Eyes abound in the animal kingdom. Some are as large as basketballs and others are just fractions of a millimetre. Eyes also come in many different types, such as the compound eyes of insects, the mirror eyes of scallops, or our own camera-like eyes. Common to all animal eyes is that they serve the same fundamental role of collecting external information from light for guiding the animal’s behaviour. But behaviours vary tremendously across the animal kingdom, and it turns out this is the key to understanding how eyes evolved. In this chapter we will take a tour from the first animals that could only sense the presence of light, to those that saw the first crude image of the world, and finally to animals that use acute vision for interacting with other animals. Amazingly, all these stages of eye evolution still exist in animals living today, and this is how we can unravel the evolution of behaviours that has been the driving force behind eye evolution.
The Astonishing Diversity of Eyes
The human eye is in no way special. We share its general building plan, and the way it develops in the embryo, with the other vertebrates: mammals, birds, reptiles, amphibians, and fish (Figures 1.1(a)–(d)). The striking similarities between all vertebrate eyes tell us they date back to a single common ancestor which had a typical vertebrate eye [Reference Lamb, Pugh and Collin1]. But how is it with all other eyes in the animal kingdom, such as the eyes of the octopus, or the compound eyes of insects? It turns out these eyes differ in fundamental ways from each other and even more so from the eyes of vertebrates, suggesting independent origins of vision in the animal kingdom [Reference Nilsson2–Reference Nilsson7]. Yet, the molecule responsible for sensing light, a form of vitamin A, seem to have been recruited for vision only once, because it is present in all animal eyes [Reference Fain, Hardie and Laughlin6, Reference Porter, Blasic, Bok, Cameron and Pringle8–Reference Ramirez, Pairett, Pankey, Serb and Speiser10]. Before we try to solve this apparent contradiction, we will first complicate the matter further by presenting a short survey of the various eye types found among invertebrate animals.
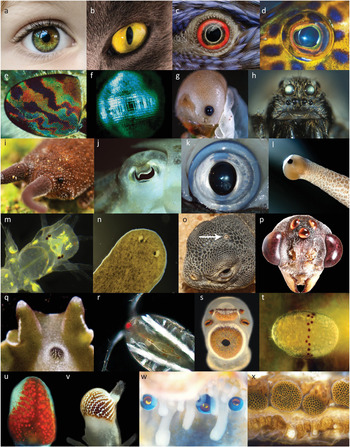
Figure 1.1 The diversity of animal eyes: (a)–(d), camera-type eyes of vertebrates: human, cat, bird (parrot), and fish (coral cod); (e), insect (horse fly) compound eye; (f), crustacean (prawn) compound eye; (g), camera-type eye in an insect (sawfly) larva; (h), multiple pairs of camera-type eyes in a wolf spider; (i), low-resolution simple eye in a velvet worm; (j) and (k) camera-type eyes in cuttlefish and squid; (l), low-resolution simple eye in a snail; (m), two pairs of low-resolution simple eyes in a juvenile ragworm; (n), lensless cup-eyes in a flatworm; (o), single parietal eye in the midline of a lizard head; (p), group of three median eyes (dorsal ocelli) between the compound eyes of a bull ant; (q), the median compound eye in a marine flatworm; (r), directional photoreceptors in the midline of a copepod crustacean; (s), two low-resolution simple eyes and four lensless cup-eyes on a sensory club of a box jellyfish; (t), directional photoreceptors in a ring around the waist of a box jellyfish larva; (u), compound eye at the arm tip of a starfish; (v), compound eye on a tentacle of a fan worm; (w), concave mirror eyes along the mantle edge of a scallop; (x), compound eyes along the mantle edge of an ark clam.
Among the most prominent eyes of any animals are those of cephalopods (octopus, squid, and cuttlefish, Figures 1.1(j) and (k)). These are animals with large eyes and excellent vision. There is a lens focusing a sharp image on a retina, just as in our own eyes. Although the shape of the lens and the eyeball differs from that of a human eye, it is practically indistinguishable from the eyes of fish or other vertebrates using vision under water. Vertebrates and cephalopods obviously share the same optical principle and functional eye design. But there are ancient differences. All parts of the cephalopod eye develop from the skin, whereas vertebrate eyes develop mainly from the brain, with only the lens and cornea originating from the skin. The retinas of cephalopod and vertebrate eyes thus have different origins. A consequence of this is that the axons sending information to the brain sensibly leave the back of the retina in cephalopod eyes but exit in the opposite direction in vertebrate eyes. This is why our retina is facing backwards, and why we need a blind spot where the axons can leave the eye.
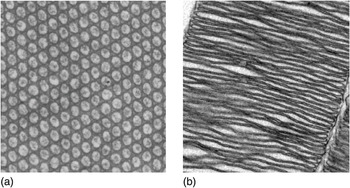
The differences between cephalopod and vertebrate eyes are even more fundamental. The photoreceptor cells that detect the light are called rods and cones in vertebrate eyes. A close inspection of these cells reveal that they keep the visual pigment in a structure derived from a cilium. Normally, cilia are tiny motile hairs used to propel cells or the liquid around them, but in rods and cones, cilia have become immotile and strongly modified into large stacks of membrane filled with visual pigment. The cephalopod retina does not have rods and cones, but photoreceptor cells of a completely different kind. Here, it is thousands of tiny membrane fingers, microvilli, that house the visual pigment in structures called rhabdoms. Microvilli without visual pigment are common in many other cells where a large surface area is needed. Consequently, photoreceptor cells of cephalopods and vertebrates have recruited two unrelated structures for elaborating the cell membrane, cilia and microvilli (Figure 1.2). Cilia use microtubules to shape the membrane, whereas microvilli use actin filaments. Thus, cilia and microvilli are fundamentally different solutions for membrane elaboration, meaning that the photoreceptor cells in vertebrate and cephalopod eyes have independently evolved the ability to pack large amounts of visual pigment in elaborate membrane structures. Rods and cones of vertebrate eyes are referred to as ciliary photoreceptors, whereas cephalopod eyes have photoreceptors referred to as rhabdomeric (rhabdoms are made of densely packed microvilli with visual pigment in the microvillar membrane).
There can be no doubt that vertebrate and cephalopod eyes have reached the same optical solution from very different origins. What about other animals? Are there more types of photoreceptor cells? It turns out that nearly all animal photoreceptors can be classified as either ciliary or rhabdomeric. The compound eyes of insects and crustaceans (Figures 1.1(e) and (f)) have rhabdomeric photoreceptors, but here the optical solution is radically different from that of cephalopod and vertebrate eyes. In compound eyes there is one lens for each pixel in the image. It is a common misconception that insects see as many images as they have facets in their eyes. That is not so. Insect vision is composed of pixels, just as human vision is. But the way light reaches the array of pixels to form an image is fundamentally different between compound eyes and simple eyes such as the camera-type eyes of vertebrates and cephalopods.
Insects and crustaceans are examples of arthropods. But not all arthropods have compound eyes. Spiders, for instance, have four pairs of camera-type eyes, and some insect larvae have a single pair of simple lens eyes (Figures 1.1(g) and (h)). Snails also have a pair of simple lens eyes, whereas rag worms have two pairs of such eyes, and planarian flatworms have one or more pairs of lens-less simple eyes (Figures 1.1(l)–(n)). The eyes mentioned so far are paired organs placed on the sides of the head, but there are also median, unpaired, eyes such as the parietal eye of lizards, the dorsal ocelli (a type of lens eyes) of insects, located between the compound eyes, or the median compound eyes present in some marine flatworms (Figures 1.1(o)–(q)).
There are eyes with even more unusual positions. Box jellyfish have no head, but well-developed lens eyes on four positions between the tentacles (Figure 1.1(s)). Starfish have compound eyes at their arm tips, and fan worms have compound eyes on their feeding tentacles (Figures 1.1(u) and (v)). Scallops have hundreds of eyes with mirror optics looking out along the shell opening (Figure 1.1(w)). Other clams have a row of lens-less compound eyes in the same position (Figure 1.1(x)). Chitons have simple eyes sprinkled all over their back, and sea urchins are covered in dispersed photoreceptors that make the entire body act as a large compound eye.
These examples give an idea of the enormous diversity of eyes in animals. Eyes can be simple or compound, have lenses or no lenses, use rhabdomeric or ciliary photoreceptors, be paired or median, and placed on the head or elsewhere [Reference Land, Nilsson, Warrant and Nilsson11–Reference Cronin, Johnsen, Marshall and Warrant13]. It is this diversity we have to account for when trying to reconstruct how eyes evolved. Initially it may seem to complicate the matter, but this diversity in fact holds the key to the origin of eye evolution.
How Did It All Start?
A single question will lead us on the right track: is there any cell type present in all eyes, from the simplest to the most advanced, that could be useful on its own, without all the other parts of the eyes? The answer is yes, the photoreceptor cells could obviously serve important functions on their own. Without the presence of any ocular structures, they could tell when it is day and when it is night, which is crucial for selecting the best time for different behaviours and for setting the biological clock. For primitive animals in water, photoreceptor cells are also essential for sensing their depth in the sea, in order to be where food is most plentiful, and for avoiding excessive levels of harmful ultraviolet light close to the surface.
Most animals still have such a sense, which just measures the levels of ambient light, and often use it to control the levels of sleep hormone, melatonin. Photoreceptor cells with this function are often located in the brain. We have such cells in our eye – possibly because it is too dark in our brain – but they are not the rods and cones we use for vision. This sense is called non-directional light sensitivity because it is based on measuring ambient light levels irrespective of the directions light comes from [Reference Nilsson5, Reference Nilsson7, Reference Foster and Hankins14].
The first animals would have been very small creatures that either crawled or swam with cilia. They are likely to have had a chemical sense and a tactile sense to orient them towards suitable places in the environment. If they had any light sensitivity it must have started as non-directional sensing of light. Even though this was a very long time ago, evolution has left clues that now allow us to reconstruct these first steps on the way to eyes and vision. By far the most important clue is the mechanism by which light is detected. In all animal eyes this is done by a very special type of receptor proteins located in the cell membrane. These receptor proteins are called opsins [Reference Porter, Blasic, Bok, Cameron and Pringle8–Reference Ramirez, Pairett, Pankey, Serb and Speiser10], where rhodopsin is the version found in the rods of our own retina.
Opsins in turn, are part of a huge family of signalling proteins called G-protein-coupled receptor proteins (GPCRs) that exist in animals, fungi, and protists but not in bacteria. Their general function is to detect specific chemicals and forward the information to the rest of the cell [Reference Fain, Hardie and Laughlin6, Reference de Mendoza, Sebe-Pedros and Ruiz-Trillo15]. There are thousands of different types of GPCRs that specifically bind to and detect a huge range of different chemicals. Opsins bind to derivatives of vitamin A, and it is the vitamin A molecule that is sensitive to light. A bent isoform of Vitamin A, called 11-cis-retinal, can absorb the energy in a single particle of light, a photon, which flips the molecule into its straight isoform, all-trans-retinal. Each opsin molecule can hold a single vitamin A molecule in a pocket and signals to the cell when the vitamin A changes from its bent to its straight isoform. This means we actually sense light by chemoreception, or, in other words, our eyes detect light by tasting a photoproduct of vitamin A.
Fundamental similarities of all animal opsins reveal that they originated once in the very early phases of animal evolution. Just to confuse the matter, there are other opsins around. Some fungi have been reported to have opsins, but these are unrelated to animal opsins. Bacteria also have molecules called opsins, but these do not belong to the GPCRs. Thus, the unique family of animal opsins tells us that this mode of light sensitivity arose very early in animal evolution [Reference Colley and Nilsson16].
The closest GPCR relative to animal opsins is the melatonin receptor, i.e. a GPCR that detects the presence of the sleep hormone melatonin [Reference D’Aniello, Delroisse, Valero-Gracia, Lowe and Byrne17, Reference Tosches, Bucher, Vopalensky and Arendt18]. This is interesting because melatonin levels are controlled by light detection with opsins. We know that melatonin receptors predate the first animal because they are found also in protists. It is possible and even likely that melatonin receptors served as light receptors before opsins evolved [Reference Nilsson7]. The reason is that melatonin is itself light sensitive and is destroyed by oxidation when illuminated. With a constant synthesis of melatonin, the concentrations would thus be high at night and low during the day. In such a simple system the melatonin receptor would have served as an ancient light receptor predating the opsins. But melatonin-based light receptors suffer from several limitations. Because melatonin is destroyed by light, new melatonin would constantly have to be synthesised, and changes in the light intensity during the day would be hard to detect because of low melatonin levels. Melatonin is also primarily sensitive to ultraviolet light, which is never as bright as the longer, visible, wavelengths.
The fact that melatonin receptors are the closest relatives to opsins suggests that it was a modification of an ancient melatonin receptor that gave rise to the first opsin [Reference Nilsson7]. By changing from binding melatonin to binding vitamin A aldehyde, the receptor became a far more efficient light receptor. Whereas melatonin is destroyed by light, vitamin A aldehyde just flips from bent to straight, and this can easily be flipped back such that the molecule can be recycled indefinitely. This is not only more economical, but also allows automatic changes of sensitivity such that small differences of intensity can be discriminated under both dim and bright conditions. Modifications of the binding pocket inside the opsin protein also allow changes of the wavelength sensitivity over a large range from ultraviolet to red. There are thus numerous benefits of replacing melatonin with vitamin A, and the first opsin would have been a major improvement in light detection.
A close look at the phylogenetic relationship of melatonin receptors and opsins among the major groups of animals reveals some interesting facts (Figure 1.3). Very shortly after the first animal opsin evolved there was a major radiation into different families of opsins, supporting the idea that the first opsin opened up new possibilities, which were rapidly exploited. Sponges were the first animal group to split from the rest of the animals, and sponges do not have any opsins – but they do have melatonin receptors (Figure 1.3). This implies that the first opsin evolved in animals after the split between sponges and other animals but before other animals diverged in the present major groups. Being able to pinpoint opsin origin that precisely also allows a reliable timing of this event. By comparisons of animal evolution, which can be reliably timed on the basis of the entire genome [Reference Dohrmann and Wörheide19], we can work out when the first opsin evolved (Figure 1.4). This turns out to have been about 800 million years ago, and it was the starting point of an evolutionary journey towards eyes and vision.

Figure 1.3 The divergence of opsins in major animal groups.
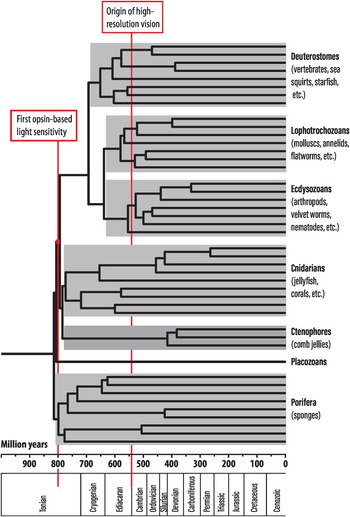
Figure 1.4 A timed phylogeny of major animal groups. Geological periods are indicated below the time scale.
What Happened after the First Opsin Evolved?
Melatonin receptors would have been adequate for telling day from night, suggesting that the rapid radiation of opsins shortly after their introduction enabled new functions that had not been possible with melatonin receptors. What might these new functions have been? There is only so much a non-directional photoreceptor can be used for. Reading the general ambient intensity could inform about the time of day, the water depth, or possibly a sudden shadow. A photoreceptor cell could be used for entirely different tasks if it provided information about how light is distributed in the environment. Such information would allow movement towards bright or dark parts of the environment (phototaxis), and greatly enhance an animal’s ability to find optimal positions in the environment [Reference Nilsson5, Reference Nilsson7]. Although non-directional photoreceptors are unable to provide guidance for phototaxis, it only takes some dark shielding pigment to make a photoreceptor cell directional (Figure 1.5(b)).
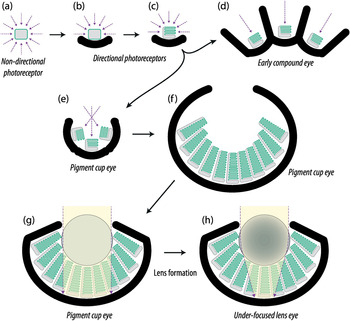
Figure 1.5 Schematic drawings of major stages in the evolution of photoreception, starting with non-directional photoreception (a), directional photoreception without membrane stacking (b), the same with membrane stacking (c), a low-resolution compound eye (d), a low-resolution cup eye (single-chambered eye) (e), a larger version of a low-resolution cup eye (f), a cup eye with a protective vitreous mass filling the cavity above the photoreceptors (g), and a more elaborate low-resolution eye where the vitreous mass had turned into a lens to produce an under-focused lens eye (h).
The combination of a photoreceptor cell and a dark pigment cell is a common feature in many of the small, wormlike animals that make up much of the animal kingdom (see an example in Figure 1.6). In bilaterally symmetric animals, such combinations typically occur as paired organs in the frontal part of the animal. By turning the head or body, directional photoreceptors are made to scan the environment and can guide the animal towards brighter or dimmer parts of the environment. Directional photoreceptors can also be used to determine what is up and down for control of body posture. Such optical statocysts are known from many invertebrates, often as unpaired median organs (e.g. Figures 1.1(p)–(r)). Finding optimal locations and body orientations in the environment would have been major competitive advantages offered by the introduction of directional photoreceptors.
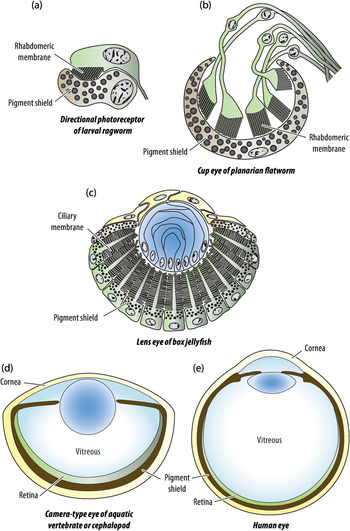
Figure 1.6 Examples of the morphology of different stages in eye evolution. Colours indicate photoreceptor cells or retina (green), pigment screen (brown, black granules), optics (blue), and external protective tissue (yellow). The directional photoreceptor (a) and the low-resolution eyes (b) and (c) are all less than 0.1 mm in diameter, whereas typical high-resolution eyes, (d) and (e), are 10–1,000 times larger (1 mm–10 cm).
To change a non-directional photoreceptor into a directional one only takes some screening pigment, but, in order to optimise the directional photoreceptor to its new tasks, a few additional modifications will be required. The typical task of non-directional photoreceptors is to detect the daily light intensity slowly changing over a huge range of some 100 million times (eight orders of magnitude) from bright sunlight to a moonless night. Scanning a directional photoreceptor cell to guide phototaxis is a very different task where the speed must be very much faster [Reference Randel and Jekely20], and the range of intensities to be discriminated varies over a much smaller range of two orders of magnitude from the dimmest to the brightest direction within a scene. But the position of this restricted range slides up and down the daily variation of eight orders of magnitude. This means the photoreceptor would have to speed up its response massively and also introduce some mechanism of shifting its gain to cope with the large variations of light intensity at dusk and dawn, and under different weather conditions [Reference Nilsson and Bok21].
Basically, the photoreceptor cell would have to change from detecting slow changes of absolute intensity to discriminating much faster changes of relative intensity within a scene. For a photoreceptor cell informing about the time of day or depth in the sea, adaptation would be detrimental, but, for comparing intensities at different directions in the environment, adaptation is a necessity. The distinction between adapting and non-adapting receptor cells is not unique to light receptors: most tactile receptors would need an adaptation mechanism, although adaptation would defeat the purpose of a receptor for noxious heat. The types of molecular machinery behind these two modes of operation are likely to have originated in other senses before they appeared in photoreceptors. An important conclusion from this is that the same photoreceptor cell cannot inform about the time of day and also guide phototaxis. There is thus a need for different types of photoreceptors to support different parts of more complex behaviours, and this is a likely reason for the early radiation of opsin types (Figure 1.3). In the next section we shall discover another principle that had a major influence on the evolution of photoreceptor cells.
A Problem of Photon Shortage Guides Evolution
Photoreceptor cells can detect light only by absorbing it, and then light behaves as particles, called photons. A consequence of this is that the detected brightness is in principle based on the number of photons contributing to the neural signal in photoreceptor cells. Even at a constant light intensity, the photons arrive randomly, very much like drops of rain, and repeated attempts to determine the intensity will result in slightly varying photon counts. More photons in each count will result in a more precise knowledge about how bright it is. The phenomenon is known as photon noise, and it sets a limit to the smallest differences in light intensity that can be discriminated. As an example, if the sensory task is to signal the arrival of dawn, the photoreceptor cell must be able to detect an increase in light intensity that exceeds the photon noise. The slowness of intensity changes means that photoreceptor cells can respond very slowly, effectively counting photons over 10 minutes or more. Then it can be calculated that a small photoreceptor cell with opsin densely packed in its membrane will count enough photons to just discriminate the intensity change from starlight to the first light of dawn [Reference Nilsson7].
This reasoning can now be used as a tool to understand eye evolution. If we calculate the ambient intensity that is needed for a directional photoreceptor to guide an animal to a dark shelter (negative phototaxis), we will find that the conditions are very different from the previous case of spotting the first light of dawn. Intensities at different directions within an environment at any given time differ much less than the huge change between day and night. Consequently, much smaller differences in intensity must be discriminated, and this requires more photons in each of the samples that are to be compared. But, to guide movement, the cell must be very much faster, with an integration time in the range of a second (almost three orders of magnitude faster than the change at dusk and dawn). On top of this, the directionality of the photoreceptor allows it to pick up light only from within a restricted angle. If we do the numbers, it turns out that the phototaxis task would work only down to mid dusk on a sunny day on land [Reference Nilsson7]. At a few metres depth in coastal water it would barely work on an overcast day, and at 10 metres depth it would not work at all. There are simply not enough photons in natural habitats to allow discrimination of light in different directions, using the same photoreceptor that we know can signal the onset of dawn.
To cure the directional photoreceptor of its shortage of photons, light collection must become more efficient. The main problem is that no more than 0.02 per cent of the light will be absorbed on passing through a membrane packed with opsin molecules. The remaining light, 99.8 per cent, will be lost by the photoreceptor cell. Clearly, this provides an opportunity for major improvements by simply folding the membrane into stacks with numerous layers. This is exactly what we find in most directional photoreceptors. Rather than spreading the opsin over a non-specialised membrane, these photoreceptor cells confine their opsin to heavily elaborated membrane structures. With the possibility of thousands of membrane layers, a photoreceptor cell can improve its sensitivity by absorbing much of the light reaching it. These membrane elaborations are evolutionary responses to the shortage of photons caused by the introduction of directional photoreception.
Ancient Alternative Solutions
As we recall, there are two major ways to elaborate the cell membrane, cilia and microvilli, and these existed well before any photoreceptor cells evolved in animals. The distinctions between cilia and microvilli are both functional and structural. Cilia are originally motile structures supported by microtubules, whereas microvilli are cell surface extensions supported by actin filaments. Since they are based on two different types of cytoskeleton, cilia and microvilli are distinct structures with no intermediates. Because photoreceptor cells with membrane elaborations are of two basic types, using either cilia or microvilli to hold the opsin, we can conclude that directional photoreception must have evolved independently at least twice, generating ciliary and rhabdomeric (microvillar) photoreceptors (Figure 1.2).
This conclusion is further supported by the fact that specific families of opsin proteins are associated with cilia and microvilli, respectively. There is thus a distinct class of ciliary opsins (c-opsins) found in ciliary photoreceptors and an equally distinct class of rhabdomeric opsins (r-opsins) found in rhabdomeric photoreceptors. Also, the molecular transduction mechanisms responsible for sensitivity adjustment (light/dark-adaptation), which became necessary in the first directional photoreceptors, are of fundamentally different types in ciliary and rhabdomeric photoreceptor cells [Reference Fain, Hardie and Laughlin6].
When these photoreceptor types were first described by electron microscopy, it was thought that vertebrates had ciliary photoreceptors and invertebrates had the rhabdomeric type [Reference Eakin and Autrum22]. But exceptions were soon discovered, and it eventually turned out that all major animal groups possess both types of photoreceptor cells [Reference Nilsson2–Reference Nilsson4, Reference Arendt, Tessmar-Raible, Snyman, Dorresteijn and Wittbrodt23], except sponges, which have no opsin-based photoreceptors at all [Reference Vopalensky and Kozmik24]. It follows that both types of photoreceptors, ciliary and rhabdomeric, evolved independently as directional photoreceptors in a common animal ancestor after sponges had split off (see Figure 1.3). From a timed phylogenetic tree of animals (Figure 1.4), we can pinpoint the life of this last common ancestor to shortly after 800 million years ago.
The Origin of Eyes
With both non-directional and directional photoreceptors (Figures 1.5(a)–(c)), the early animal ancestor could use light to control a range of simple behaviours such as regulating activity according to the daily light cycle, regulating the animal’s depth in water, moving towards or away from light, or orienting the body to the up–down gradient of light intensity. Because each new function required specific performance properties of the photoreceptor cells, this evolution led to several distinct types of photoreceptor cells, each specialised for a particular task. In the more simply organised animals that remain today, such as jellyfish, each behaviour that is controlled by light appears to have its own group of photoreceptor cells in its own specific place [Reference Land, Nilsson, Warrant and Nilsson11, Reference Nilsson and Bok21, Reference Picciani, Kerlin, Sierra, Swafford and Ramirez25]. Only later was light detection centralised into specific organs – eyes.
So how did eyes evolve from the photoreceptors we have discussed so far? The step is actually rather short. Directional photoreceptors operate by guiding the animal towards dimmer or brighter directions to find the optimal position in the environment. The animal can do this only by continuously turning its body until it aims in a direction with a desired light intensity. To find the overall light gradients in the environment, the photoreceptor must pick up light from a very large angle, roughly a hemisphere, which also leads to limited accuracy in finding the best direction. Improving this situation requires narrower angles of sensitivity, which in turn requires much more scanning to cover all angles. This problem is easily solved by multiplying the directional photoreceptor and having the photoreceptors point in different directions (Figure 1.5(d)). This also abolishes the need for the scanning body movements since information about the intensity in different directions can be obtained by comparing the signals from the different photoreceptors. Such spatial resolution within a group of photoreceptors is the basic definition of an eye.
If we use terminology from digital cameras, each directional photoreceptor with a unique field of view corresponds to one image pixel. It is obvious that a small number of directional photoreceptors will provide only a very crude image of the environment, but it is more accurate than the single-directional photoreceptor, scanning is no longer necessary, and, most importantly, resolution can be improved by just continuing to multiply the number of photoreceptors and correspondingly narrow their angle of sensitivity. Complicating factors are that a new type of neural mechanism comparing neighbouring photoreceptors must be introduced, and the behavioural control must change from scanning to steering. This calls for centralised neural control – a brain. Elaborations of the developmental genetics controlling cell position and orientation must, of course, also be introduced at this point. A beautiful recapitulation of the transition from directional photoreception to spatial vision, and the first formation of a brain, can be seen in larval stages of modern ragworms [Reference Randel and Jekely20].
Two Solutions to Imaging
Multiplying directional photoreceptors to make a low-resolution image immediately forces a choice between two alternatives. The photoreceptors can form a common cup or point away from one another (Figures 1.5(d) and (e)). The common cup alternative will lead to a single-chambered eye, and the other alternative will lead to a compound eye. We know from the occurrence of eyes in different animal groups today that both directions were initiated numerous times independently. The single-chambered eyes of jellyfish, molluscs, and vertebrates originated independently from directional photoreceptors, as can be judged from the use of different types of opsins, different transduction mechanisms, development from different tissues, and the use of different principles for membrane stacking. The same is true for compound eyes in groups such as insects, fan-worms, and starfish, which also show convincing evidence of having independently evolved from different types of directional photoreceptors [Reference Nilsson and Bok21]. But, before we continue to the sophisticated eyes of vertebrates, cephalopods, and insects, we shall ask some important questions about the low-resolution eyes that evolved from directional photoreceptors.
When Did the First Eye See the World?
Because eyes obviously evolved independently many times from directional photoreceptors, it is not easy to work out which animal group was the first to take this step. It could even be that the first animals with eyes belong to a group that became extinct long time ago and left no traces. But if we assume that the first eye evolved within an animal group that still exists today and that their present eyes date back to the first eye, then the phylogenetic tree of Figure 1.4 can give us some indication. Because the eyes in different animal groups differ in terms of being compound or single-chambered, using rhabdomeric or ciliary photoreceptors, etc., we can conclude that eyes must have evolved after the last common ancestor of groups that independently acquired eyes, but before the split of groups that share the same type of eye. Using this kind of reasoning, the first eyes probably arose sometime between 650 and 550 million years ago. Then there are also many groups where eyes evolved much later.
What Are Low-Resolution Eyes Used For?
In every group where eyes evolved, the first eyes must have started with just a small number of photoreceptor cells (Figures 1.5(d) and (e) and 1.6(b)), and consequently been able to resolve an image with only a small number of pixels. For finding a suitably lit environment, this is still better than just a non-directional photoreceptor. But, as soon as the multiplication of units was initiated, it would be developmentally simple to keep adding new units to bring up the number of photoreceptor cells and thus the number of image pixels. It would seem that once this process was initiated, high-resolution vision would evolve rapidly. But in most branches of the animal kingdom that possess eyes today (about half of the roughly 30 animal phyla), all but three still have only low-resolution eyes with anything between 10 and a couple of hundred photoreceptor cells. These eyes are tiny, less than a millimetre across, and not very conspicuous.
Clearly, low-resolution vision must be very useful and there must be reasons not to continue eye evolution beyond a certain level of acuity. Because examples of these low-resolution eyes are so abundant, their roles have now been studied in several animal groups such as jellyfish, flatworms, velvet worms, and starfish, and the outcome is simple and straightforward [Reference Nilsson and Bok21, Reference Nilsson, Gislén, Coates, Skogh and Garm26–Reference Kirwan, Graf, Smolka, Mayer, Henze and Nilsson30]. Low-resolution eyes are used for finding a suitable habitat and for positioning of the animal within that habitat. For these tasks, it is sufficient to see large stationary structures in the environment. Low-resolution vision, i.e. a few hundred pixels and less, is adequate for moving about, avoiding collisions with objects, finding a free path, moving in and out of shelters, finding areas where food is likely to be found by other senses, or keeping a straight course (an excellent strategy to get out of an unfavourable environment). Resolution (acuity) of a few hundred pixels or less is not good enough to see other animals at any distance beyond immediate reach. Low-resolution eyes therefore cannot be used to see prey, predators, or individuals of the same species.
What animals with low-resolution vision are able to see appears poor indeed to us, but is perfectly adequate to support the visually guided behaviours of these animals. In Figure 1.7, vision in a velvet worm is shown as an example. These worms are found in the southern hemisphere. They belong to the phylum Onychophora, which is a sister group of the arthropods. Velvet worms are active at night and hunt for insects using touch and smell. They cannot see their insect prey at distances beyond immediate reach. At dawn they have to find a moist shelter under a fallen log, or else they will dry out and perish before the day is over. Their tiny eyes will guide them to suitable shelters at dawn, and also guide them back out to suitable hunting grounds at dusk [Reference Kirwan, Graf, Smolka, Mayer, Henze and Nilsson30].

Figure 1.7 High-resolution human vision compared with low-resolution vision in a velvet worm. Colour vision is common among the high-resolution eyes of vertebrates and insects, whereas low-resolution eyes typically are colour-blind. The low-resolution vision of the velvet worm is good enough for finding fallen logs or other shelters (simulation by Mikael Ljungholm).
The Problem of Photon Shortage Returns: The Evolution of Lenses
In single-chambered eyes, resolution can be improved by adding photoreceptors, making the eye cup deeper, and making the aperture smaller (as illustrated in Figures 1.5(e) and (f)). In compound eyes the number of units (ommatidia) must grow, and the photoreceptors must be set in correspondingly deeper pigment tubes. In both cases, the visual angle of each photoreceptor must shrink as the number of photoreceptors grows. When the animal moves, this leads to motion blur unless the integration time is also made shorter. On top of this, lower-contrast image details become of interest as resolution increases.
We recall that the transition from non-directional to directional photoreception was associated with a sensitivity problem that was solved by creating stacks of membrane to catch a larger fraction of the light that reaches a photoreceptor cell. Now, as the resolution of the first eyes was increasing, more photons needed to be detected by each photoreceptor during each integration time (to see lower contrasts), but the narrower angles and shorter integration times have the opposite effect. The consequence of this is that improvement of resolution by adding more units requires an increasingly brighter world for the eye to function.
To some extent the need for better sensitivity can be met by deeper stacks of photoreceptor membrane, such that these structures turn into long rods. But this is not enough to bring resolution up to more than 10–50 pixels, and photon shortage would make vision impossible at dusk or at more than a few metres depth in coastal water. Better resolution and better sensitivity require a new invention. The answer is to concentrate light by focusing, or in other words to make a lens in front of the photoreceptors [Reference Nilsson5, Reference Nilsson7]. In single-chambered eyes there is obvious space for a lens in the eye cup, and this may anyway be filled by vitreous cells protecting the eye from damaging ultraviolet light or providing structural support (Figures 1.5(g) and 1.6(c)). If these cells produce large amounts of protein, they will form a lens that concentrates light on all photoreceptors in the eye and cures the photon shortage. The lens need not be perfectly focused on the layer of photoreceptors (the retina; see Figure 1.5(h)). For low-resolution vision partial focusing will suffice. In compound eyes, increasing resolution leads to large numbers of extremely long pigment tubes that have to be perfectly straight. Here too lenses offer an excellent solution, which allows narrow visual angles in much shorter ommatidia.
Without lenses, eyes would not be able to evolve beyond the first extremely low-resolution versions still found in flatworms today (Figures 1.1(n) and 1.6(b)). Most other low-resolution eyes have some sort of lenses (Figures 1.5(h) and 1.6(c)). It is important to note that the primary purpose of the lenses in low-resolution eyes is not to make sharp images, but to bring sensitivity up to levels needed for vision at the naturally occurring light intensities on planet Earth.
From Low to High Resolution and the Birth of a New Ecological System
Increasing the resolution such that each pixel covers only a few degrees means that other animals can be spotted at distances larger than a few body lengths. When this threshold was reached for the first time, a new ecological era was initiated. With an instantaneous long-range sense, predation becomes a lucrative business. And it is easily made even more effective by continuing to improve resolution. But here, the problem of photon shortage strikes for the third and last time. When membrane stacking has been fully exploited and lenses have been introduced, there is only one way left to increase sensitivity beyond that of low-resolution vision. That way is simply to make the eye larger (Figures 1.6(d) and (e)). A larger lens has a larger area picking up light, and this will compensate for the smaller pixel angles in high-resolution vision. The great thing here is that this strategy has no upper limit. If the eye is large enough, any kind of resolution can be obtained at any light intensity. But the growth in eye size from the sub-millimetre low-resolution eyes must be substantial to enable long-range spotting of other animals or seeing at very low intensities. Accordingly, high-resolution eyes measure from a few millimetres to more than 25 cm in present-day animals. The largest eyes, with a diameter of 27 cm, belong to deep-sea giant squid inhabiting depths of 1,000 metres in the ocean [Reference Nilsson, Warrant, Johnsen, Hanlon and Shashar31].
The required growth in eye size may have been the main obstacle for adopting a visually guided predatory lifestyle. The lack of obvious animal fossils older than 540 million years suggests that earlier animals were small and soft-bodied like many of the present-day animals that have only low-resolution vision. But growing large eyes to get resolution high enough for seeing other animals is not entirely straightforward. High resolution itself means that the nervous system will have to process information from a very large number of photoreceptor cells. And, to see other animals, there has to be a sizeable brain with new types of neural circuits that can detect small moving objects against a cluttered background [Reference Wiederman, Shoemaker and O’Carroll32]. For this information to be useful, the animal will also have to evolve means of efficient locomotion with muscles and a skeleton to attach them to, and of course motor circuits in the brain that can orchestrate adequate behaviours in response to the new visual information. All of this together means that a large, mobile animal with complex behaviours would have to evolve from a small, slow-moving animal with much simpler behaviours. In terms of evolutionary change, this is quite a revolution.
For the species that underwent this change, it would have implied an entirely new and very active lifestyle. For the ecological system, the first visually guided predator would have introduced completely new selective pressures on other species that tried to avoid ending up on the dinner plate. Potential evolutionary responses would have been to develop equally good vision and efficient mobility to escape the predators, starting an arms race with vision and mobility. Other alternatives would be to develop protective armour, or change to a burrowing lifestyle to avoid the visually guided predators. All these evolutionary innovations would have left obvious traces in the fossil record. And they did indeed. The event called the Cambrian explosion, about 540 million years ago, displays everything expected from the introduction of visually guided predation: the first macroscopic animals with prominent eyes, fins, and walking appendages, internal as well as external skeletons for muscle attachment, protective shells, body armour, and spikes, and the first deep-burrowing animals. All of these appear suddenly in the fossil record from the early Cambrian era, suggesting that it really was a major turnover forcing the entire ecological community to undergo a dramatic change (Reference Nilsson2, Reference Parker33, Reference Zhao, Bottjer, Hu, Yin and Zhu34). All just because some animals went from low-resolution vision to high-resolution vision.
Most of the early Cambrian fossil animals appear to have had an exoskeleton and compound eyes, but that may be a false bias because they are more prone to form fossils than other types of animals and types of eyes. Even the vertebrate eye probably arose in the early Cambrian, whereas cephalopods may have acquired their high-resolution eyes a little later. The rest of the animal kingdom kept their low-resolution eyes, and still have them today. Many of these animals are tiny and inconspicuous, which of course helps in a world full of visually guided predators. We still live in the ecological system that started 540 million years ago. In principle, not much novelty has been introduced in vision for the past 500 million years, even though animals have in that time evolved into many new forms and conquered the land. Some later cases of new eye evolution have occurred in animals such as clams and fan worms (Figures 1.1(v)–(x)), where the original eyes were lost and non-directional shadow detectors were recruited to form new eyes for predator detection [Reference Nilsson35–Reference Bok, Porter, Ten Hove, Smith and Nilsson37].
To sum up this long story, eye evolution followed a series of four steps: non-directional light sensitivity, directional light sensitivity, low-resolution vision, and finally high-resolution vision. In three specific phases, photon shortage intervened in the evolutionary process and led to membrane stacking, lenses, and large eyes. The evolutionary process leading to eyes started when the first opsin evolved about 800 million years ago and reached the final stage of high-resolution vision some 540 million years ago. The first opsin protein evolved in a common ancestor after sponges had split off the animal family tree. Except for the origin of opsins, all the major steps of eye evolution have occurred multiple times, and some rough counts are presented in Figure 1.8. It was the competition for efficient behaviours that drove evolution from simple light sensing to acute vision. Photoreception and vision have been pivotal for the evolution of animals, and even our own existence is a consequence of eye evolution.
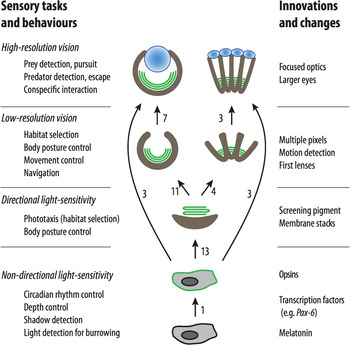
Figure 1.8 A schematic diagram of the four key stages in eye evolution. The stages of evolution of new behaviours are listed to the left, and associated innovations and changes are listed to the right.
The discovery of identical control genes for eye development (Pax-6) in vertebrates and insects [Reference Gehring and Ikeo38] was originally taken as evidence for a single origin of all animal eyes, but overwhelming evidence to the contrary [Reference Nilsson2–Reference Nilsson7, Reference Land and Nilsson12, Reference Colley and Nilsson16, Reference Vopalensky and Kozmik24, Reference Picciani, Kerlin, Sierra, Swafford and Ramirez25, Reference Bok, Capa and Nilsson36, Reference Nilsson and Arendt39] clearly demonstrates that each of the transitions between the four key stages has occurred independently numerous times (a conservative count of the number of independent transitions is indicated next to the arrows in Figure 1.8). The need for opsin expression in specific cells is the obvious reason for conserved developmental genes.