LEARNING OBJECTIVES
-
Understand the varying degrees of interrelatedness of the concepts of autism and childhood schizophrenia and how these concepts and their relationship has evolved over time
-
Understand the distinctive and overlapping features of ASD and schizophrenia, including clinical, cognitive and neurobiological aspects
-
Appreciate the most significant diagnostic and management challenges in clinical settings in patients with a combination of ASD and psychotic experiences
In 1893, the German psychiatrist Emil Kraepelin described, among endogenous psychoses, dementia praecox as a ‘peculiar simple condition of mental weakness of subacute development occurring at a youthful age’ (Reference KraepelinKraepelin 1893). First manifestations of this condition would typically occur during young adulthood, although a group of individuals presented with a very-early-onset form of the disorder. There followed a steady rise in the number of individual case reports of juvenile psychosis, including children with ‘dementia praecocissima’ (Reference De SanctisDe Sanctis 1909) or ‘dementia infantilis’ (Reference HellerHeller 1908). In 1911, the Swiss psychiatrist Eugen Bleuler made two important contributions in this regard (Reference BleulerBleuler 1911). First, he coined the term ‘schizophrenia’ to replace Kraepelin's ‘dementia praecox’. Second, he described in these patients a ‘turning inward into the own world’ and a denial of contact, a symptom that he named ‘autism’. For a long time autism was therefore considered a symptom of schizophrenia, and children with early forms of dementia praecox were reclassified as patients with ‘childhood schizophrenia’ (Reference LutzLutz 1937).
It was not until 1943 that Leo Kanner described a particular subgroup of children among those with psychosis who, unlike the majority who would have had at least 2 years of normal development, had had difficulties in establishing relationships since the beginning of their lives (Reference KannerKanner 1943) and thus presented with ‘extreme autistic isolation’ (hence he called it ‘infantile autism’). Independently and almost in parallel (in 1944) the Austrian psychiatrist Hans Asperger described a group of children with similar characteristics and coined the term ‘autistic psychopathy’. His work remained largely unknown until it was cited in a publication by Reference WingLorna Wing in 1981 (Reference WingWing 1981).
During the 1950s and 1960s, and despite Kanner's delineation of ‘infantile autism’ as a separate entity, the terms ‘autism’, ‘childhood schizophrenia’ and ‘childhood psychosis’ were used to describe the same children, depending on the tradition of the institution where the diagnosis was established. Besides, American and European diagnostic classification systems such as DSM-I (1952), DSM-II (1968), ICD-7 (1955) and ICD-8 (1967) still conceptualised autism as a form of child psychosis. It was not until 1972 that, based on Kolvin's studies (Reference Kolvin, Ounsted and HumphreyKolvin 1971), the differential value of Kanner's autism was recognised by Michael Reference RutterRutter (1972). Autism and childhood schizophrenia were acknowledged as separate entities and, as a result, in DSM-III (1980): (a) the category of autism was included but, to avoid confusion between the schizophrenia symptom ‘autism’ and the disorder described by Kanner, the term ‘pervasive developmental disorder’ (PDD) was also introduced; and (b) ‘schizophrenia childhood-type’ disorder was removed and lumped together with the adult forms of schizophrenia. ICD-9 (1978) also recognised autism and childhood schizophrenia as separate diagnostic entities, but kept the term ‘psychoses with origin specific to childhood’ to refer to infantile autism, childhood disintegrative disorder and other atypical or unspecified childhood psychosis.
The autism/schizophrenia distinction was maintained in subsequent editions of the DSM and ICD. Over the 1990s and through DSM-III-R (1987), ICD-10 (1992) and DSM-IV (1994), there was a further refinement of increasingly complex criteria for autistic disorder/PDD that resulted in a broadening of the concept of autism. Indeed, in 1979, Lorna Wing introduced the concept of an ‘autism continuum’, and 9 years later Allen coined the term ‘autism spectrum disorder’ (Reference AllenAllen 1988), which took precedence over PDD in many fields and was subsequently used in DSM-5 (2013).
A summary of the conceptualisation of schizophrenia and autism spectrum disroder (ASD) through the history can be found in Box 1.
BOX 1 Historical conceptualisation of autism spectrum disorder and early-onset schizophrenia
Before Kanner
-
Reference KraepelinKraepelin (1893): dementia praecox
-
Reference HellerHeller (1908): dementia infantilis
-
Reference De SanctisDe Sanctis (1909): dementia praecocissima
-
Reference BleulerBleuler (1911):
-
‘schizophrenia’ to replace dementia praecox
-
‘autism’ as a symptom of schizophrenia
-
-
Early forms of dementia praecox = ‘childhood psychosis’ (‘childhood schizophrenia’, following publication of DSM-I in 1952)
Reference KannerKanner (1943)
-
‘Infantile autism’, among those with childhood psychosis
-
In parallel, Asperger introduces ‘autistic psychopathy’
After Kanner
-
1950–1970: ‘autism’ = childhood schizophrenia/psychosis
-
Reference Kolvin, Ounsted and HumphreyKolvin et al (1971) and Reference RutterRutter (1972): the differential value of Kanner's autism
-
Reference Wing and GouldWing (1979): ‘autism continuum/spectrum’
-
DSM-III (1980): autism and schizophrenia as separate entities:
-
pervasive developmental disorders (PDD)
-
schizophrenia and related disorders
-
-
Reference Weinberger, Nasrallah and WeinbergerWeinberger (1986) and Reference Murray and LewisMurray & Lewis (1987): schizophrenia = neurodevelopmental disorder
-
Reference AllenAllen (1988): autism spectrum disorders (ASD)
-
DSM-5 (2013): ASD and schizophrenia spectrum disorders (SSD)
The ‘broadening’ of the diagnostic criteria for autism in the past three decades led to further discussions of the boundaries of psychosis and autism. In parallel, the idea of psychosis/schizophrenia being part of the larger group of neurodevelopmental disorders (Reference Weinberger, Nasrallah and WeinbergerWeinberger 1986; Reference Murray and LewisMurray 1987) brought to the fore again the discussion about schizophrenia and autism having a variety of overlapping features and about the possibility that these disorders represent final pathways for common causal factors and pathophysiological processes (Reference Watkins, Asarnow and TanguayWatkins 1988). Findings of schizophrenia and autism research reinforced this emerging debate. Although several lines of genetic evidence supporting the distinction between childhood-onset schizophrenia and autism initially emerged (Reference Sporn, Addington and GogtaySporn 2004), further evaluation of all studies suggested some common genetic abnormalities associated with both disorders (Reference Rapoport, Chavez and GreensteinRapoport 2009). From the conceptual point of view, it could be argued that, even though psychosis and autism are indeed distinct neurodevelopmental disorders, there might be a degree of pathophysiological overlap yet to be fully defined giving rise to clinical characteristics that are similar but follow diverging developmental trajectories. Autistic characteristics can be present both before and after the diagnosis of psychotic disorders (Reference Sporn, Addington and GogtaySporn 2004; Reference Tsakanikos, Sturmey and CostelloTsakanikos 2007; Reference Mouridsen, Rich and IsagerMouridsen 2008) and a proportion of children with ASD present with psychotic-like symptoms (Reference Sullivan, Rai and GoldingSullivan 2013; Reference Kyriakopoulos, Stringaris and ManolesouKyriakopoulos 2015).
Boundaries and overlaps
Epidemiology
Worldwide prevalence estimates for schizophrenia range between 0.5 and 1% (Centers for Disease Control and Prevention 2013). Less than 15% of patients with schizophrenia are diagnosed with their first episode before the age of 18 years (Reference Cannon, Jones and HuttunenCannon 1999; Reference Amminger, Henry and HarriganAmminger 2011) and the disorder is very rare indeed in childhood (0.1–1% of all schizophrenic disorders manifest before age 10) (Reference Remschmidt, Schulz and MartinRemschmidt 1994). Although a higher proportion of boys among earlier-onset cases has been reported, this finding has not been consistent (Reference Werry, McClellan and AndrewsWerry 1994; Reference McClellan, McCurry and SnellMcClellan 1999).
With regard to ASD, the most recent report from the Centers for Disease Control and Prevention (2014) found that ASD was affecting 1 in every 68 children aged 8 years. In recent years, there has been ongoing debate over whether the prevalence of this disorder is indeed increasing (Reference WeintraubWeintraub 2011; Reference Baxter, Brugha and ErskineBaxter 2015). One of the challenges in ASD epidemiological studies is that there is a dearth of data beyond childhood and adolescence (Reference Baxter, Brugha and ErskineBaxter 2015). An additional challenge is establishing and studying age at onset, as it can be difficult to detect ASD in very young children.
Developmental aspects, continuity and co-occurrence
Since the first studies by Rapoport and colleagues, numerous authors have shown that a significant proportion of children and adolescents with psychotic experiences or schizophrenia fulfil criteria for ASD or present with marked developmental abnormalities during childhood (Reference Alaghband-Rad, McKenna and GordonAlaghband-Rad 1995; Reference Eggers, Bunk and KrauseEggers 2000; Reference Sporn, Addington and GogtaySporn 2004; Reference Sprong, Becker and SchothorstSprong 2008; Reference Rapoport, Chavez and GreensteinRapoport 2009; Reference Bevan Jones, Thapar and LewisBevan Jones 2012). Of note, pre-existing ASD is present in as many as 30–50% of patients with childhood-onset schizophrenia and may appear many years before schizophrenia is diagnosed (Reference Rapoport, Chavez and GreensteinRapoport 2009). The same applies to individuals with adult-onset schizophrenia (Reference Mouridsen, Rich and IsagerMouridsen 2008; Reference Unenge Hallerback, Lugnegard and GillbergUnenge Hallerback 2012; Reference Selten, Lundberg and RaiSelten 2015).
Phenomenology
Differing features
As traditionally described, autism and schizophrenia are quite distinct and show unique characteristics, with different symptom profiles, course of illness and family histories (Reference RutterRutter 1972). With regard to clinical features, the main differences between the two disorders include: (a) different age at onset (very early onset in autism v. typically late-adolescent/young-adult onset in schizophrenia); and (b) different symptom profiles, as individuals with autism typically show few, if any, positive symptoms of schizophrenia (Reference Rumsey, Andreasen and RapoportRumsey 1986; Reference Konstantareas and HewittKonstantareas 2001; Reference Spek and WoutersSpek 2010).
Overlapping features
With regard to overlapping features, premorbid and early negative symptoms of schizophrenia can sometimes be indistinguishable from symptoms of autism. Early social withdrawal, flattened affect, poor eye contact, communication problems and restricted speech, odd behaviours and psychomotor abnormalities might be among those shared symptoms. However, in schizophrenia, there is often a noticeable exacerbation of pre-existing developmental deviance in the year or two before the first psychotic episode. In keeping with this, some authors have suggested that prodromal symptoms of schizophrenia can be easily misdiagnosed as ASD (Reference Sugihara, Tsuchiya and TakeiSugihara 2008). The language difficulties in ASD and schizophrenia (e.g. restricted dialogue, poverty of speech or neologisms) can also easily be confused (Reference Kyriakopoulos and FrangouKyriakopoulos 2007).
Furthermore, deficits in higher-order social cognition, including social reciprocity and theory of mind, which are hallmarks of ASD (Reference Baron-CohenBaron-Cohen 1989), can also be present in schizophrenia (Reference FrithFrith 1992). Both groups often show difficulty in recognising social cues, in understanding irony, humour, metaphors and proverbs, and in identifying emotions or intentions in other people. In social situations these difficulties can lead to confusion, paranoid interpretations and inappropriate reactions that can make it difficult for these individuals to forge and sustain friendships or social relationships (Reference Mazurek and KanneMazurek 2010; Reference Harley, Boardman and CraigHarley 2012). They can also show deficits in awareness of self and others and in the ability to make an accurate evaluation of reality, of the emotional content of social scenes/situations and of their inner states (Reference Sasson, Tsuchiya and HurleySasson 2007; Reference WilliamsWilliams 2010; Reference Touskova and BobTouskova 2015). Nevertheless, studies have also found different patterns of social cognition abnormalities between the groups and it has even been suggested that these disorders exhibit diametric deficits, with theory of mind being underdeveloped in autism spectrum conditions and overdeveloped in psychosis (Reference Crespi and BadcockCrespi 2008; Reference Bara, Ciaramidaro and WalterBara 2011). This, however, is not supported by newer studies.
Both patients with ASD and those with schizophrenia can show odd mannerisms, stereotyped behaviours and a rigid, inflexible pattern of thinking and behaving (Reference Delahunty, Morice and FrostDelahunty 1993; Reference RidleyRidley 1994; Reference Leung and ZakzanisLeung 2014). Catatonia has been described as a common endophenotype for both ASD and schizophrenia and there are descriptions of children presenting with mixed forms of catatonia, autistic and psychotic symptoms (Reference Shorter and WachtelShorter 2013).
Mixed presentation
Finally, there is a group of children that present with a mixed ASD/psychotic phenotype. Reported psychotic experiences have been associated with pre-existing ASD and ASD traits in a large cohort (Reference Sullivan, Rai and GoldingSullivan 2013). It is also known that anxiety can induce formal thought disorder (usually considered a symptom of psychosis) even in typically developing children, but more so in those with ASD (Reference Solomon, Ozonoff and CarterSolomon 2008). However, high levels of anxiety did not provide an adequate explanation for psychotic-like symptoms in ASD in an in-patient sample of children (Reference Kyriakopoulos, Stringaris and ManolesouKyriakopoulos 2015). Other disorders, such as obsessive–compulsive disorder and depression (also relatively common in ASD; Reference Simonoff, Pickles and CharmanSimonoff 2008), can give rise to symptoms that may look psychotic in nature. Similarly, mood lability and disinhibition associated with ASD may pose diagnostic challenges in deciding on the co-occurrence of bipolar disorder. Detailed discussion of these possible comorbid conditions goes beyond the scope of this article. The construct of multiple complex developmental disorders (MCDD) used to describe children who present with ASD symptoms and also exhibit affective dysregulation and disordered thinking (Reference Cohen, Paul and VolkmarCohen 1986; Reference Buitelaar and Van der GaagBuitelaar 1998) is an interesting model for the study of mixed phenotypes of that nature (Reference Rapoport, Chavez and GreensteinRapoport 2009; Reference Kyriakopoulos, Stringaris and ManolesouKyriakopoulos 2015).
Cognitive deficits and neurological soft signs
ASD and schizophrenia show shared cognitive deficits, including impaired executive function and cognitive flexibility, abstract reasoning and goal-directed problem-solving behaviours, as well as impaired general functioning as measured with the IQ (Reference Pennington and OzonoffPennington 1996; Reference Tiihonen, Haukka and HenrikssonTiihonen 2005; Reference Mayes and CalhounMayes 2008). Neurological soft signs (e.g. poor sensory integration, motor coordination or sequencing of complex motor acts) are a vulnerability marker in schizophrenia, reflecting abnormal brain maturation (Reference Bombin, Arango and BuchananBombin 2005), but individuals with ASD have also been noted to display sensorimotor impairments (Reference Halayem, Bouden and AmadoHalayem 2009). One study compared the prevalence of neurological soft signs in patients with Asperger syndrome or early-onset psychosis and healthy controls and found no significant differences between the two groups of patients in their neurological soft signs profile (Reference Mayoral, Merchan-Naranjo and RapadoMayoral 2010).
Neuroimaging findings
Neuroimaging studies have reported abnormal structure and function of brain regions associated with social cognition both in individuals with ASD (Reference Pelphrey, Adolphs and MorrisPelphrey 2004) and in those with psychosis (Reference Bertrand, Achim and HarveyBertrand 2008). For example, volumetric grey matter deficits (Reference Giedd, Raznahan and Alexander-BlochGiedd 2015), microstructural white matter changes (Reference Dwork, Mancevski and RosoklijaDwork 2007; Reference Kates, Ikuta and BurnetteKe 2009) and abnormal brain gyrification (Reference Kates, Ikuta and BurnetteKates 2009; Reference Palaniyappan and LiddlePalaniyappan 2012) have been reported as early vulnerability markers for both disorders. However, few neuroimaging studies have directly compared brain structure or function between these patient groups (Reference Pinkham, Hopfinger and PelphreyPinkham 2008; Reference Toal, Bloemen and DeeleyToal 2009; Reference Radeloff, Ciaramidaro and SiniatchkinRadeloff 2014). Indeed, the main available information on the neurological overlaps and differences between ASD and schizophrenia comes from reviews and meta-analyses that put together findings from studies separately comparing each group with healthy controls (Reference Abdi and SharmaAbdi 2004; Reference Cheung, Yu and FungCheung 2010; Reference Sugranyes, Kyriakopoulos and CorrigallSugranyes 2011). For example, functional magnetic resonance imaging (fMRI) studies show how both ASD and schizophrenia patients shared activation deficits in similar brain regions within the ‘social brain network’ (e.g. hypoactivation in the superior temporal sulcus while performing a theory of mind task), together with deficits unique to each condition (e.g. hypoactivation of thalamus unique to schizophrenia) (Reference Sugranyes, Kyriakopoulos and CorrigallSugranyes 2011).
Genetics
There is increasing evidence showing genetic links between schizophrenia and autism. Family studies have shown an increased risk of ASD among individuals whose parents or siblings have been diagnosed with schizophrenia or bipolar disorder (Reference Sullivan, Magnusson and ReichenbergSullivan 2012). Besides, single nucleotide polymorphisms (SNPs) located in various candidate genes (such as DISC1 or NRXN1) have yielded a handful of genetic associations that have been reported in both disorders (Reference Morris, Kandpal and MaMorris 2003; Reference Kilpinen, Ylisaukko-Oja and HennahKilpinen 2008). However, recent studies also showed overlap between five investigated major psychiatric disorders, with the overlap being the least extensive for autism and schizophrenia (Cross-Disorder Group of the Psychiatric Genomics Consortium 2013).
Furthermore, an overlap has been reported between copy number variants (CNVs) found in ASD and in schizophrenia (e.g. 15q duplication, 22q11 or 22q13 deletion) (Reference Rapoport, Chavez and GreensteinRapoport 2009), especially in genes involved in neurodevelopmental pathways – e.g. those involved in the regulation of oligodendrocyte and synaptic functions and myelination (Reference Vourc'h, Martin and Bonnet-BrilhaultVourc'h 2003; Reference Voineskos, de Luca and BulginVoineskos 2008). Although CNVs have been found to be very frequent in ASD (up to 20% in individuals with intellectual disability; Reference Shen, Dies and HolmShen 2010) and to confer high associated risk for the disorder, it is currently thought that common variants also play a great role in ASD, particularly in non-syndromic ASD. In the case of schizophrenia, the heritability is mainly explained by common variance, although rare variants may cause some cases. There is also some evidence that de novo mutations in the same genes (e.g. SHANK3) could account for a number of cases of schizophrenia (Reference Gauthier, Champagne and LafreniereGauthier 2010) and ASD (Reference Durand, Betancur and BoeckersDurand 2007). Although these mutations with high penetrance account for a small proportion of cases, they might provide converging pathophysiological trajectories for schizophrenia and ASD.
Some genetic abnormalities that increase the risk of either disorder in particular cases may not be very specific and have a pleiotropic nature with different behavioural outcomes. This is the case, for example, with 22q11 or 22q13 deletions. Besides, both disorders share environmental risk factors (e.g. higher parental age, intrauterine infections, maternal stress or maternal immune disruption) which, in the interplay with genetics, might be leading to different and time-sensitive changes in neuronal maturation, migration, synaptic integrity and neurotransmitter functions (Reference Meyer, Feldon and DammannMeyer 2011).
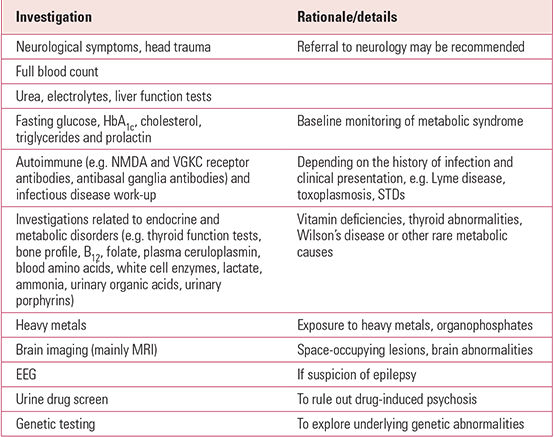
Diagnostic investigations
The aetiology of ASD or psychosis is typically not clear and, in fact, the phenotype of schizophrenia or autism is probably due to the interaction between several genetic risk factors and external contributors, leading to aberrant neurodevelopment. In any case, a specific aetiological factor can rarely be found but some medical work-up is warranted.
In young people with psychosis or ASD, particularly at first onset, neurological and medical conditions that may cause or contribute to the clinical presentation must be always considered. In very-early-onset schizophrenia, in particular, because of its rarity, the need to rule out other rare medical and genetic conditions is paramount. Central nervous system insults, autoimmune and infectious diseases, endocrine and metabolic disorders will all need to be explored. Similarly, intoxication with heavy metals such as lead needs to be considered. Among other examples, 40% of individuals with childhood onset of Wilson's disease, which is associated with abnormalities in copper metabolism, present with neuropsychiatric symptoms (Reference Hancu, Mihai and AxeleradHancu 2011), including psychotic and autistic symptoms. Epilepsy may also be an underlying and comorbid disorder in both psychosis and ASD, which warrants further assessment of clinical presentation and potential episodes and neurophysiological investigations with the use of electroencephalography (EEG). Undiagnosed brain tumours, while uncommon, must also be considered, which warrants detailed neurological examination and possibly investigation with MRI.
Genetic testing of individuals with ASD or early-onset psychosis should also be considered. In the case of schizophrenia, studies show that onset in childhood is relatively likely (10% of cases) to be associated with underlying genetic abnormalities (Reference Addington and RapoportAddington 2009). It is difficult to establish specific recommendations and they tend to be quickly outdated owing to the rapid developments in the field. Currently, in most cases without a specific clinical phenotype that guides to a more targeted analysis, array comparative genomic hybridisation (CGH) or exome sequencing are the tools of choice in both ASD and childhood-onset schizophrenia. Testing for fragile-X syndrome would also be recommended in all children presenting with psychosis and commonly in children with ASD, especially in those with significant cognitive deficits (Reference Freitag, Staal and KlauckFreitag 2010).
A summary of the recommended baseline investigations for patients with early-onset psychosis and ASD can be found in Table 1.
TABLE 1 Investigations that should be considered at baseline assessment for early-onset psychosis and autism spectrum disorder
Implications for diagnosis and treatment/management strategies
Current classification systems organise ASD and schizophrenia as mutually exclusive diagnoses. Their conceptualisation as neurodevelopmental disorders, in addition to the fact that there are overlaps in symptomatology and cognitive deficits, may prevent clinicians from detecting the potential co-occurrence or continuity of autism and schizophrenia. This means that if an individual develops autism as a young child, they may be less likely to receive a diagnosis of schizophrenia later on, even if psychotic symptoms emerge. Therefore, knowledge about ASD/schizophrenia similarities and distinct characteristics is warranted to inform differential diagnosis and diagnosis of comorbidity. In some patients, it may be more appropriate to take a longitudinal perspective, bearing in mind deviant neurodevelopment with changing predominant clinical features and different intervention needs at different points in life.
With regard to treatment options, psychosocial, behavioural and educational interventions have been used with individuals with early-onset schizophrenia (Reference Puig, Penades and BaezaPuig 2013) and those with ASD (Reference Seida, Ospina and KarkhanehSeida 2009), mainly to facilitate social integration or to improve cognitive skills, with some positive outcomes. Regarding pharmacological strategies, core symptoms of ASD have not been shown to respond to medication, although it can be helpful in the treatment of comorbid psychiatric conditions (Reference Young and FindlingYoung 2015). Second-generation antipsychotics (SGAs) are the first-line option for schizophrenia at any age (Reference Stafford, Mayo-Wilson and LoucasStafford 2015). However, although they may be effective in reducing the positive symptoms of schizophrenia (e.g. delusions and hallucinations), they have limited effects on negative symptoms and cognitive impairment (Reference Miyamoto, Miyake and JarskogMiyamoto 2012). SGAs are also effective in managing certain symptoms, particularly irritability and aggression, associated with ASD (Reference Young and FindlingYoung 2015). When antipsychotics are used in any condition, assessment of the potential benefits needs to be balanced against the risks of side-effects (Reference Schneider, Papachristou and WimberleySchneider 2015; Reference Stafford, Mayo-Wilson and LoucasStafford 2015).
Emerging evidence points towards some promising pharmacological interventions that may be useful in treating both ASD and psychotic disorders, since they aim at tackling symptoms such as social and communication impairments or negative symptoms, which are associated with both conditions. They include, among others, oxytocin, cholinesterase inhibitors and glutamatergic drugs, although the evidence base supporting their use in clinical practice is still developing (Reference Erhart, Marder and CarpenterErhart 2006; Reference Posey, Erickson and McDouglePosey 2008).
Case vignettes
Three brief fictitious case vignettes of children with mixed ASD and psychotic features are presented below, highlighting the diagnostic and management challenges they may pose. In all cases, choice of out-patient or in-patient treatment will depend on clarity of clinical presentation, support in the community and identified risks.
Case vignette 1
A 9-year-old girl with mild premorbid developmental abnormalities in the form of slightly delayed motor milestones and language acquisition, reciprocal social interaction difficulties, school underperformance and mood dysregulation presents with a 4-month history of increased anxiety, suspiciousness that her parents are trying to poison her, sleep disturbance and reported auditory and visual hallucinatory experiences of unclear nature. Her parents report that she seems distractible and apparently talks to herself and responds to unseen stimuli, but on direct questioning the girl denies any unusual experiences.
This is likely to be very-early-onset psychosis. Detailed diagnostic assessment of early development, contextual factors, family history of psychosis, and the patient's psychiatric comorbidities will assist in deciding whether another explanation of her symptoms may be more likely. Detailed physical examination, including clinical assessment of possible epilepsy and investigations mentioned above (MRI of head, EEG and array CGH) should follow. A referral to a child neurologist may be indicated. She is very likely to score positive for ASD in a semi-structured interview such as the Autism Diagnostic Observation Schedule, but this would not necessarily mean she meets criteria for ASD. A semi-stuctured developmental interview for autism (such as the Autism Diagnostic Interview – Revised) will carry more weight in that respect. A diagnosis of ASD would not exclude psychosis, as developmental abnormalities can be conceptualised as part of the developmental trajectory of either disorder. Modifications in her environment or schooling either before or alongside other interventions will probably be necessary. A trial of a low-dose antipsychotic is a reasonable option if alternative explanations for her presentation (e.g. anxiety or depression) are thought to be less likely.
Case vignette 2
A 12-year-old boy with a previous diagnosis of Asperger syndrome presents with a 3-month history of extreme anxiety, social withdrawal, thoughts of self-harm, school refusal and bizarre ideas about ‘aliens’ controlling his actions. In primary school he was a big fan of science fiction and was very popular among his peers, but he seems to have lost interest in social relationships since his transfer to a mainstream secondary school.
In this case, careful consideration should be given to all alternative formulations. A better understanding of how the symptoms emerge and are related to the boy's previous circumscribed interests is necessary. Physical assessment and investigations will depend on what has been previously conducted and on the most likely explanation for his current clinical presentation. It is likely that it is a result of extreme anxiety associated with a school environment not meeting his needs and an increase in social demands affecting his attempts to make sense of inner states. Increased support at school or alternative schooling need to be considered. If anxiety is indeed felt to be the most likely cause of his symptoms, psychological interventions (e.g. cognitive–behavioural therapy) and a trial of a selective serotonin reuptake inhibitor with or without a low-dose antipsychotic could be initiated. Very intensive monitoring of clinical response, risks and side-effects of medication is paramount.
Case vignette 3
A 16-year-old boy with a previous diagnosis of moderate intellectual disability and ASD presents with a 2-month history of a sudden change in behaviour, including increased body-rocking movements, episodes of unresponsiveness, insomnia, aggression, unusual experiences of hearing his name being called and seeing a man in black, and believing that children from his school are ‘out to get him’.
This boy will benefit from a detailed physical assessment and detailed investigations, including an assessment for epilepsy. A referral to a child neurologist is likely to be necessary. Psychiatric assessment will be similar to those in the previous vignettes. If the change in his behaviour is not related to an acute physical problem or significant change in his environment, his older age makes it more likely for his clinical presentation to be psychosis than it would in younger children. Of course, more common alternative psychiatric explanations will also need to be explored. Treatment with antipsychotic medication will probably need to be tried. Other interventions in relation to his education will depend on his school setting and levels of support.
Conclusions
ASD and schizophrenia are two distinct behavioural outcomes of aberrant neurodevelopment and their differentiation is frequently easy, clinically useful and in accordance with current categorical diagnostic systems. However, the boundaries are not always clear and several lines of evidence from phenomenology, epidemiology, genetics and neuroscience point towards a close relationship between the two disorders. Their overlapping characteristics and potential co-occurrence might pose important diagnostic challenges in clinical practice. Understanding the ASD/schizophrenia overlaps, boundaries and uncertainties may help clinicians to revisit and better understand the relationship between the two disorders and inform more effective management strategies.
MCQs
Select the single best option for each question stem
-
1 Current research indicates that the proportion of individuals with childhood-onset schizophrenia who meet criteria for ASD during childhood is:
-
a 0.1–2%
-
b 2–8%
-
c 9–20%
-
d 20–30%
-
e 30–50%.
-
-
2 Research shows that the core social communication deficits of people with ASD can be effectively managed using:
-
a risperidone
-
b selective serotonin reuptake inhibitors
-
c clonidine
-
d naltrexone
-
e none of the above.
-
-
3 The following deficits have been associated with schizophrenia:
-
a deficits in theory of mind
-
b social isolation
-
c deficits in executive function
-
d b and c
-
e a, b and c.
-
-
4 Risk factors that are shared between schizophrenia and ASD include:
-
a brain injury
-
b 22q11 deletion
-
c bullying
-
d cannabis use
-
e all of the above.
-
-
5 In concluding on the diagnosis of psychosis/schizophrenia against a background of ASD it is necessary to take into consideration:
-
a age at onset
-
b presence of previous developmental abnormalities
-
c presence of organic conditions
-
d a and b
-
e a, b and c.
-
MCQ answers
| 1 | e | 2 | e | 3 | e | 4 | b | 5 | e |




eLetters
No eLetters have been published for this article.