Approximately 0.5–1% of older adults live with bipolar disorder,Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters1 which will place significant and increasing demands on healthcare services. The UK National Institute for Health and Care Excellence (NICE) bipolar disorder clinical guideline2 states that older people with bipolar disorder should be offered the same range of treatments as younger adults. Such treatments may not be appropriate because of significant differences in the nature of bipolar disorder in later life. Bipolar disorder in older adults differs in presentation, is more complex, and is accompanied by high rates of physical comorbiditiesReference Lala and Sajatovic3 and poorer cognitive function even during euthymia,Reference Gildengers, Mulsant, Begley, Mazumdar, Hyams and Reynolds Iii4 which may significantly affect psychosocial outcomes.Reference Kohler and Marlinge5 As cognitive functioning declines, older adults with bipolar disorder may struggle to apply effective coping responses to difficult situations,Reference Gildengers, Mulsant, Begley, Mazumdar, Hyams and Reynolds Iii4 including adopting more passive coping styles than non-clinical older adults.Reference Schouws, Paans, Comijs, Dols and Stek6 Despite these differences, few studies have evaluated psychological interventions developed for older people with bipolar disorder. In the USA, a psychosocial skills training programme for older people with various mental health problems (schizophrenia, schizoaffective disorder, depression and bipolar disorder) improved community living skills, functioning and self-efficacy, and reduced psychiatric symptoms.Reference Bartels, Forester, Mueser, Miles, Dums and Pratt7 Additionally, medication adherence skills training led to improvements in medication adherence and management, depressive symptoms and quality of life for older people with bipolar disorder.Reference Depp, Lebowitz, Patterson, Lacro and Jeste8 However, it is not clear from the first study what the outcomes are specifically for older people with bipolar disorder and neither study targeted personal recovery, which is highlighted in national policy9 and NICE guidelines.2
Over the past four decades, the personal recovery movement, led by patients, has called for a new approach to define ‘recovery’. This has led to a shift from recovery outcomes being based predominantly on eradicating symptoms and ‘cure’, and a move toward building strength and resilience in an individual, enabling them to take control of their life and mental health problems. This paper reports on a recovery-focused therapy (RfT) intervention for bipolar disorder, adapted to meet the needs of older adults (RfT-OA). Research for working-age adults has shown this approach is beneficial in terms of personal recovery and relapse outcomes.Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10 This is the first study to evaluate the feasibility and acceptability of RfT for older people living with bipolar disorder.
Method
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures were approved by the UK National Health Service (NHS) Ethics North West – Preston Committee process (reference 15/NW/0330). Written informed consent was provided by all participants. The study was preregistered with the ISRCTN registry (identifier ISRCTN13875321), and a study protocol informed by the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) GuidelinesReference Chan, Tetzlaff, Altman, Laupacis, Gøtzsche and Krleža-Jerić11 was pre-published.Reference Tyler, Lobban, Sutton, Depp, Johnson and Laidlaw12 The study is consistent with the Consolidated Standards of Reporting Trials (CONSORT) extension for pilot and feasibility trialsReference Eldridge, Lancaster, Campbell, Thabane, Hopewell and Coleman13 (see Supplementary File 1 available at https://doi.org/10.1192/bjo.2022.582).
Three study changes were necessary after protocol publication. Minimum age was reduced from >65 to ≥60 based on the United Nations14 definition of older adults and in line with best practice.Reference Laidlaw, Davidson, Toner, Jackson, Clark and Law15 The latest version of the Structured Clinical Interview for DSM-5, research version (SCID-5-RVReference First, Williams, Karg and Spitzer16) was used. Outcome assessments were not conducted blind to allocation because of resource constraints.
Objectives
The study aim was to evaluate the feasibility and acceptability of RfT-OA in an randomised controlled trial design. The first objective was to determine the feasibility of RfT-OA in terms of whether clinicians would refer older adults into a randomised controlled trial; whether older adults would self-refer into a randomised controlled trial; whether older adults with bipolar disorder would consent to participate in an randomised controlled trial of a psychological intervention offered in addition to treatment as usual (TAU) versus TAU alone; and participant attrition rates (overall and by study arm) during assessment, intervention and follow-up. The second objective was to determine the acceptability of RfT-OA in terms of whether individuals adhered to the intervention and participants’ experiences of the intervention. Finally, with regards to future research, we aimed to identify the most appropriate primary outcome measure and estimate parameters needed to determine the sample size for a future trial.
Trial design
This was a parallel, two-armed, randomised controlled trial comparing TAU with up to 14 sessions of RfT-OA in addition to TAU, conducted across two NHS trusts in North-West England. A nested qualitative study explored the acceptability of RfT-OA.
Participants were allocated to trial arms with simple (1:1) randomisation by independent researchers at Lancashire Clinical Trials Unit, using the ‘Sealed Envelope’ randomisation programme (www.sealedenvelope.com), after baseline assessment. The study was overseen by a trial steering committee and a service user reference group.
Participants
A target of 50 participants (25 per treatment arm) was considered sufficient to obtain robust feasibility and acceptability information about trial procedures and the RfT-OA therapy, and to allow for expected attrition rates.Reference Lancaster, Dodd and Williamson17 Participants were recruited from NHS mental health services, voluntary groups, advertisements in local media and on the Bipolar UK website (https://www.bipolaruk.org), and from ‘Spectrum Connect’, a confidential database (maintained by a research team at Lancaster University) of individuals who have previously consented to being approached about research studies. Recruitment was from December 2016 to January 2018, and May 2019 to September 2019; final follow-up was in September 2020 because of the lead investigator's maternity leave. Interested individuals were screened for provisional eligibility with the Mood Disorder Questionnaire (MDQReference Hirschfeld, Williams, Spitzer, Calabrese, Flynn and Keck18). Participants aged ≥60 years from North-West England who screened positive on the MDQ were invited to a baseline interview.
Eligibility interview
Written informed consent was obtained from all participants. They were assessed with the SCID-5-RV and the Montreal Cognitive Assessment (MOCAReference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead and Collin19) to confirm eligibility criteria, and completed the observer-rated and self-report measures (see ‘Clinical and functional outcomes’ section).
Inclusion/exclusion criteria
Inclusion criteria were as follows: aged ≥60 years; SCID-5-RV diagnosis of bipolar disorder (type 1 or 2); no current episode of mania, hypomania, depression or mixed episode in the past 4 weeks; and sufficient English-language fluency to consent and take part. Exclusion criteria were an MOCA score of ≤22 or currently receiving psychological therapy.
RfT-OA
The original therapy manual was co-produced with adults with lived experience of bipolar disorder,Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10 and adapted for this study with older adults (RfT-OA). RfT focuses on helping clients to identify meaningful, personal goals to help them live well alongside their bipolar disorder experiences. These goals are not predetermined to have a clinical focus because the individual with bipolar disorder is assumed to be best placed to determine their own priorities. As such, personally defined goals can be symptom-related or focus on other areas such as relationships, social engagement or work. During the initial sessions, the therapist and client develop a shared understanding of recovery and how working toward goals that are of personal value may have a significant impact on the person's life. Developing an idiosyncratic formulation is a fundamental part of the process, ensuring the therapy approach is consistent with the person's needs. RfT highlights the importance of developing and maintaining a flexible approach to engagement during therapy, placing emphasis on building rapport and the timing, duration and frequency of sessions.
Adaptations for RfT-OA were informed by a literature review on adapting psychological interventions for older adults with mental health problems,Reference Chand and Grossberg20 and three focus groups with older people with bipolar disorder.Reference Tyler, Lobban, Long and Jones21 Adaptations included building confidence, competence and assertiveness; greater emphasis on relationship with the therapist; allocating more time to explore extensive and complex histories; and explicit acknowledgement of the wealth of experience the older person with bipolar disorder is bringing to the therapeutic relationship. Specific adaptations to enhance memory and learning included repetition and using session summaries, consistent with existing literature.Reference Chand and Grossberg20 Participants were offered 14 therapy sessions, with six or more considered an appropriate threshold for the therapeutic dose, based on the original RfT study.Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10
TAU
No changes were made to any other interventions. Psychiatric medication use and previous therapy experience were recorded.
Therapists
RfT-OA was delivered by three qualified clinical psychologists. E.T. delivered the majority of the therapy (n = 17). F.L. and S.H.J. worked with one client each. All were trained in the use of RfT-OA and attended fortnightly peer supervision.
Key outcomes: feasibility and acceptability
Information was collected on number of referrals per month, recruitment sources, number of people assessed for eligibility and provided consent. Participant retention was assessed during assessment, intervention and follow-up periods, including completion of outcome measures. Prespecified criteria were used to interpret the findings in terms of feasibility and acceptability outcomes for progression to a definitive randomised controlled trial, using a traffic light system.Reference Avery, Williamson, Gamble, O'Connell Francischetto, Metcalfe and Davidson22
Additional outcomes: feasibility and acceptability
Therapeutic alliance was assessed at the start (session three or four), middle (session eight or nine) and end of therapy (session 13 or 14), using the Working Alliance Inventory – Short Form (therapist and client forms).Reference Tracey and Kokotovic23
Clients also rated the therapy on two scales: how useful they found the therapy (from 0 (not at all) to 10 (extremely)) and whether they would recommend the therapy to a friend experiencing similar problems (from 0 (definitely not) to 10 (definitely yes)). Participants were given a copy of the scales and an envelope during their penultimate therapy session, to return at the final session.
Clinical and functional outcomes
Multiple clinical and functional outcome measures were used to assess participant attrition rates, participant views of measures from qualitative interviews and to provide preliminary data on outcomes. Participants were followed from baseline, with telephone interviews at 12, 24, 36 and 48 weeks (observer-rated measures), and by post at 24 and 48 weeks (self-report measures). The full list of observer and self-report measures can be found in Supplementary File 2.
The candidates for primary outcome measure were the Hamilton Rating Scale for Depression (HRSDReference Hamilton24), Bech–Rafaelsen Mania Scale (MASReference Bech, Rafaelsen, Kramp and Bolwig25), modified Longitudinal Interval Follow-Up Evaluation (SCID-LIFEReference Keller, Lavori, Friedman, Nielsen, Endicott and McDonald-Scott26) to assess time to relapse, and the Bipolar Recovery Questionnaire (BRQReference Jones, Mulligan, Higginson, Dunn and Morrison27), based on the original RfT study.Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10
Quality assurance
D.D. completed 18 telephone follow-up assessments when E.T. was on maternity leave. Interrater reliability between E.T. and D.D. was assessed at both baseline and follow-up.
Data analysis
Analyses were conducted with SPSS for Windows version 25 (IBM Corp.28), and followed a prespecified statistical analysis plan, approved by the trial steering committee.
Analysis was ‘as randomised’. Summary statistics were used to estimate key parameters, such as rates of recruitment, consent to the trial and retention to therapy and follow-up assessments. Linear models were used to assess the effect of RfT-OA versus TAU alone on each continuous outcome measure, with baseline value of the relevant measure as covariate. Time to first relapse was analysed with Kaplan–Meier plots and the Cox regression modelling, with time since last episode as the covariate. Separate analyses were performed for three types of relapse (any episode, depressive or manic). The focus of the analysis was point estimation with 95% confidence intervals, rather than statistical significance. For statistical models, missing data were assumed to be missing at random (dependent only on intervention group and baseline).
Pooled standard deviations for each continuous outcome, and median time to relapse, were used to estimate key parameters needed (in conjunction with data from other relevant trials, such as the study by WaltersReference Walters, Jacques, dos Anjos Henriques-Cadby, Candlish, Totton and Xian29) to determine the sample size needed for a future trial. Correlations between baseline and follow-up for the continuous outcomes were also estimated, as substantial correlations can be used to reduce the target sample size if linear modelling techniques are used for analysis.
Qualitative interviews were analysed with content analysisReference Cavanagh30 to understand participants’ experiences of key aspects of the trial process and psychological intervention. Key points and supporting quotes are summarised in the results section and Supplementary File 2.
Missing data
Missing items were imputed using a pro rata strategy, provided that at least 75% of the items were available. The exception was the World Health Organization Quality-of-Life Scale (WHOQOL-BREf31), where there is a predefined strategy for handling missing data. Please see Supplementary File 3 for details.
Results
Participants
Thirty-nine participants were recruited and randomised (see Table 1). The majority were White British (97%, n = 38) and female (67%, n = 26), with an average age of 67 (s.d. = 6) years. Most (74%, n = 29) were in the ‘young old’ age category of 60–69 years.Reference Forman, Berman, McCabe, Baim and Wei32 Most participants were diagnosed with bipolar disorder type 1 (87%, n = 34), and average age of diagnosis was 48 (s.d. = 11) years.
Table 1 Participant demographic and clinical characteristics
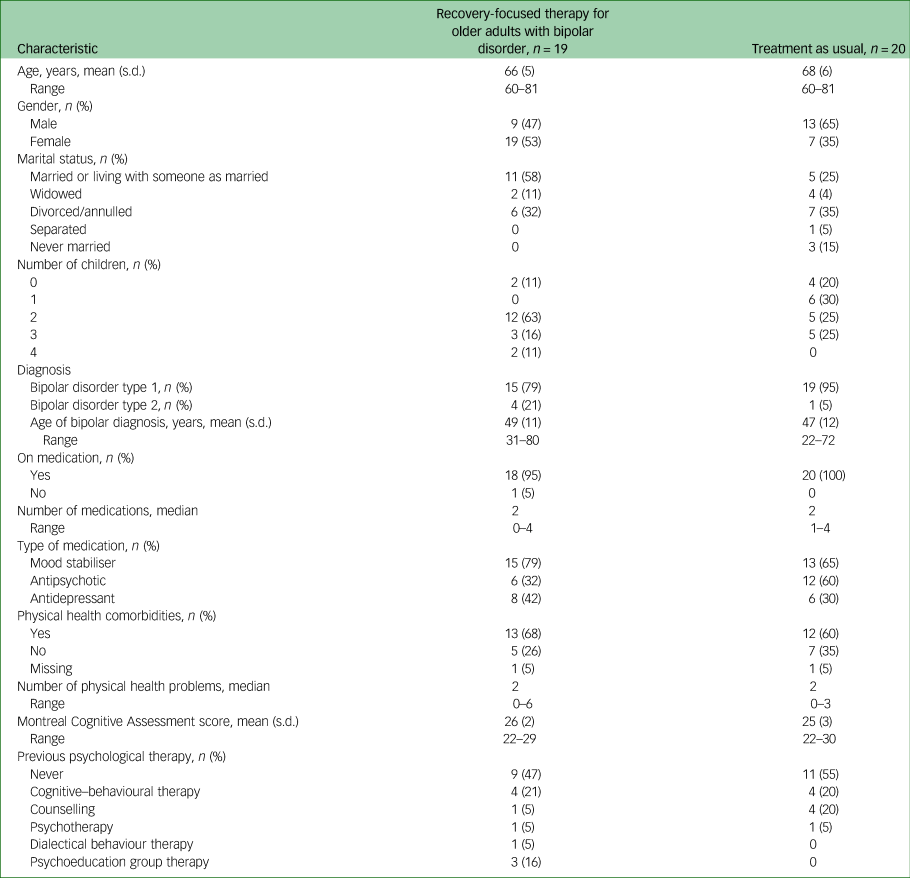
Mean MOCA score was 26 (s.d. = 2), indicating mild to no cognitive impairment.Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead and Collin19 Most participants reported physical health difficulties (64.1%, n = 25), ranging from zero to six difficulties (median of two), consistent with previous research.Reference Lala and Sajatovic3 Please see Table 1 for further participant demographics and clinical characteristics.
Participants: qualitative interviews
All participants offered RfT-OA were invited to take part in the qualitative interviews. Eight participants took part: two women and six men, aged 61–72 (mean: 65) years, and attending 7–14 sessions of RfT-OA.
Key outcomes: feasibility and acceptability
Consent, recruitment and retention
Table 2 summarises feasibility and acceptability outcomes and colour codes with a traffic light system (green, feasible; amber, feasible with modifications; red, stop). Ninety people were referred into the study, 17 by a clinician (Fig. 1). Of the 88 screened for provisional eligibility, 41 did not meet the study criteria, one refused because of physical health difficulties, and three either cancelled or did not attend their baseline interview. Forty-three out of 47 eligible participants consented to participate, although two then became unwell and two consented but then were uncontactable. Thus, 39 out of 47 eligible and consenting participants took part in the trial (green).
Table 2 Feasibility and acceptability outcomes

a. Criteria were developed by the research team (E.T., S.H.J., F.L. and C.S.), based on previous trials for individuals with bipolar disorderReference Jones, Smith, Mulligan, Lobban, Law and Dunn10,Reference Jones, Knowles, Tyler, Holland, Peters and Lobban33 and comparable studies investigating psychotherapeutic treatment for depression in later life.Reference Breckenridge, Zeiss, Breckenridge, Gallagher and Thompson34,Reference Arean, Perri, Nezu, Schein, Christopher and Joseph35
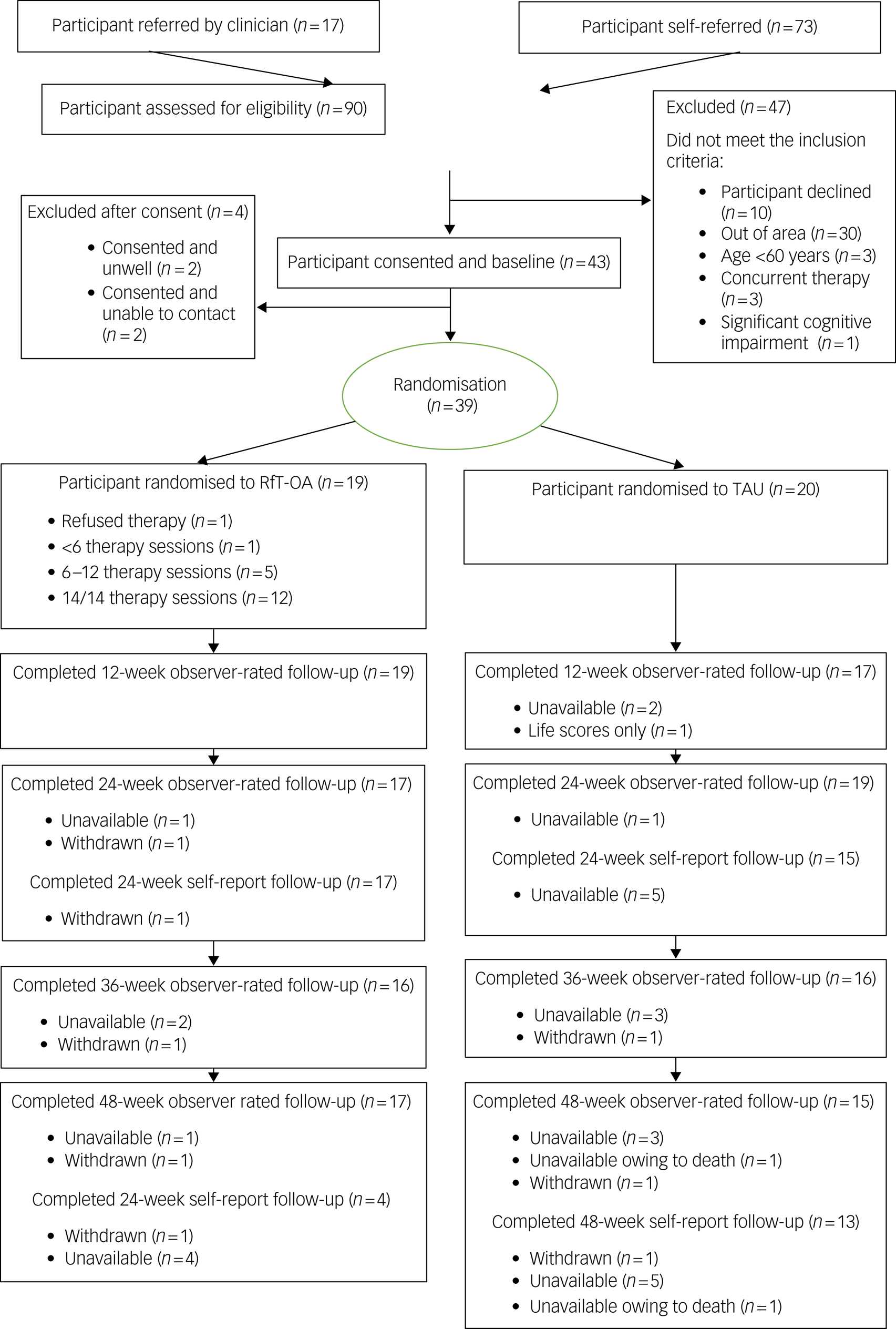
Fig. 1 Consolidated Standards of Reporting Trials (CONSORT) diagram. RfT-OA, recovery-focused therapy intervention for bipolar disorder, adapted to meet the needs of older adults; TAU, treatment as usual.
Thirty-nine participants were randomised (29 via self-referral and ten identified by a clinician) over 17 months; two participants per month (amber).
Retention for observer-rated measures was over 80% for each follow-up, with 82% (n = 32) retention at 48-week follow-up (green). Retention for the self-report measures was over 80% at the 24-week follow-up and 69% (n = 27) for the 48-week follow-up self-report (amber). One participant lost to follow-up died during the final follow-up period for reasons unrelated to the trial, as confirmed by the trial steering committee.
Eight baseline interviews were double-rated by D.D. to assess whether participants met study criteria (age, MOCA score, a bipolar disorder type 1 or 2 diagnosis and to confirm the participant was not in a current episode). Fifteen follow-up interviews were also double-rated to assess whether the participants met criteria for an SCID-5-RV episode and type of episode during a follow-up period. There was 100% agreement regarding eligibility criteria and for follow-up interviews.
Therapy retention
Mean attendance was 12.2 (s.d. = 3.3) sessions. One participant did not start therapy, 89% (n = 17) attended more than six sessions (amber) and 68% (n = 13) attended all 14 sessions (green).
Additional outcomes: feasibility and acceptability
Therapeutic alliance
Alliance data was available from 15 clients at the start of therapy (mean age 63 years, s.d. = 5), eight clients in the middle of therapy (mean age 66 years, s.d. = 5) and nine clients at the end of therapy (mean 65 years, s.d. = 7). Alliance rated by the therapists was similar, with 15 ratings at the start of therapy (mean age 64 years, s.d. = 6), eight ratings in the middle of therapy (mean age 63 years, s.d. = 7) and nine ratings at the end of therapy (mean age 65 years, s.d. = 6). Alliance ratings are similar to those observed for psychological therapy in cohorts of younger people with bipolar disorder.Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10,Reference Jones, Knowles, Tyler, Holland, Peters and Lobban33
Client ratings of therapy
Data was available for 15 participants: the usefulness of therapy score average was 9 (s.d. = 2) and the likelihood of recommending therapy score average was 9 (s.d. = 1), meaning that participants found therapy very useful and were very likely to recommend to a friend.
Qualitative interviews findings
Participants’ experiences of the intervention
The majority of participants indicated that they valued RfT-OA and highlighted its positive impact on their lifestyle, family and work relationships. One participant found it difficult to engage because of concurrent marital problems. Value was derived from both the recovery approach and learning strategies to manage emotions in a new way. The majority of participants felt that 14 therapy sessions was enough. The participant who had not felt the benefit from the sessions attended seven sessions and expressed that they would have liked more. Participants felt the session length of 50–60 min was sufficient, and valued the flexibility of the sessions being offered at home, work or the university. See Supplementary File 4 for example quotes.
Research process
On the whole, participants found the research process acceptable, although five out of eight participants indicated they would prefer the follow-up appointments to be face to face rather than on the phone. Participants were positive about receiving and returning the self-report measures by post. Participants did not express strong views on the acceptability and relevance of individual outcome measures. See Supplementary File 4 for example quotes.
Clinical and functional measures
Effect size estimation for candidate primary outcome measures
BRQ
BRQ scores in both groups were increased at the 24-week follow-up, which was numerically sustained in the intervention group and reduced in the TAU at 48 weeks (Table 3). However, there is not a clear signal of potential clinical effectiveness. Pooled s.d. was 369.4 and 359.2 at 24 and 48 weeks, respectively. The estimated correlation between the baseline and the 24-week scores was high (0.73), but there was no positive correlation between baseline and 48-week scores.
Table 3 Candidate primary outcome measures

See Supplementary File 4 for P-values. TAU, treatment as usual; RfT-OA, recovery-focused therapy for older adults with bipolar disorder; HRSD, Hamilton Rating Scale for Depression; MAS, Bech–Rafaelsen Mania Scale.
SCID-LIFE (time-to-relapse)
RfT-OA participants had fewer depressive or manic relapses (18 TAU v. 7 RfT-OA), and demonstrated a longer median time to relapse (15.5 v. 8.5 weeks) during follow-up.
Over 48 weeks, 17 participants experienced a depressive relapse (11 TAU v. 6 RfT-OA) and eight experienced a manic episode (7 TAU v. 1 RfT-OA).
HRSD
HRSD scores indicated mild depression on average in TAU at 24- and 48-week follow-up, compared with no depression in RfT-OA (Table 3). The pooled s.d. was 7.0 at 24 weeks and 7.6 at 48 weeks. The estimated correlation between the baseline and follow-up scores was very low (0.15 at 24 weeks and 0.04 at 48 weeks).
MAS
MAS scores remained low throughout, indicating low levels of mania (Table 3). MAS score was lower in the RfT-OA group at both the 24- and 48-week follow-up compared with TAU, suggesting lower levels of mania following therapy. The pooled standard deviation was 4.1 at 24 weeks and 2.2 at 48 weeks. The estimated correlation between the baseline and 24-week scores was 0.44, but there was no positive correlation between baseline and 48-week scores.
Completion rates for candidate primary outcome measures
BRQ
At baseline, 39 out of 39 participants completed the BRQ, although two questionnaires were missing ≥25% of the data; therefore 37 questionnaires were included. At 24 weeks, 31 out of 39 questionnaires were returned, with one excluded from analyses because of missing item data. At 48 weeks, 27 out of 39 questionnaires were returned, with one excluded from analyses because of missing data.
Time to relapse, HRSD and MAS
Observer-rated assessments measuring time to relapse and mood symptoms were completed at baseline, 12, 24, 36 and 48 weeks. Completion rates were 36 out of 39 participants at 24 weeks, and 32 out of 39 participants at 48 weeks, because of either participant drop-out or unavailability.
Acceptability of candidate primary outcome measures
Participants did not give any specific feedback about particular questionnaires, although they indicated that the questionnaires in general were easy to understand (see Supplementary File 4).
Additional clinical outcomes
Additional clinical outcomes are reported in Supplementary File 5, with higher mean scores observed in the Personal and Social Performance Scale in RfT-OA at both 24- and 48-week follow-up, indicating higher levels of functioning. The other clinical outcome measures tended to favour RfT-OA, although this was not consistent across all measures and time points.
Discussion
This is the first study to develop and evaluate a psychological intervention specifically for older adults with bipolar disorder, using a randomised controlled trial design. RfT-OA was developed and designed in collaboration with individuals living with bipolar disorder,Reference Tyler, Lobban, Long and Jones21 enhancing the quality, value and relevance of the study.
Feasibility and acceptability of RfT-OA
The study findings largely support the feasibility and acceptability of RfT-OA, evaluated against predefined criteria. It was possible to recruit 39 participants at a rate of 2.3 per month (amber progression zone). This was with the lead researcher as the sole recruiter, working part-time for the second half of the recruitment period. A well-resourced research team would be in a stronger position to enhance the recruitment rate (discussed further below). Retention to follow-up was strong and balanced across trial arms, with rates comparing favourably to previous bipolar disorder and older adult depression trials.Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10,Reference Laidlaw, Davidson, Toner, Jackson, Clark and Law15,Reference Scott, Paykel, Morriss, Bentall, Kinderman and Johnson36 Intervention arm participants engaged with RfT-OA, demonstrating a significant commitment to therapy. Two participants did not attend six or more therapy sessions (amber); therefore, strategies to overcome any potential barriers are discussed below. Interviews indicated that participants valued RfT-OA, corresponding with high client ratings of therapy usefulness and recommending to a friend. Alliance ratings were acceptable and comparable to younger bipolar disorder cohorts.Reference Jones, Smith, Mulligan, Lobban, Law and Dunn10,Reference Jones, Knowles, Tyler, Holland, Peters and Lobban33
Primary outcome measure
Completion rates were higher for the observer-rated measures (e.g. time to relapse and mood symptoms; 86–90%) than the self-report measures (e.g. the BRQ; 70–81%), although a number of strategies have been identified below to enhance self-report rates. Feedback from the interviews indicated that participants were positive about the data collection process and did not report any difficulties completing any of the measures. The MAS, HRSD and SCID-LIFE (time to relapse) demonstrated a signal of benefit, although the trial was not powered to test intervention effectiveness. Further consultation with older adults with bipolar disorder will be needed to identify which measure is most relevant to their experiences and what they would want to change during RfT-OA, before confirming the most appropriate and meaningful primary outcome measure for a definitive trial.
Sample size for a future trial
To help determine the appropriate sample size for a future trial, pooled standard deviations for MAS and HRSD and the median time to relapse were estimated. For the HRSD, a minimal clinically important difference in a trial context is often deemed to be around 3 points,Reference Gask, Dowrick, Dixon, Sutton, Perry and Torgerson37 with an s.d. of 7–8 points (consistent with our results). Using a conservative 8-point s.d. (standardised effect size of 0.375), a sample size of 302 would be needed to achieve 90% power (two-sided 5% significance level using a two-sample t-test), inflated to 404 (202 per group) to allow for an anticipated 25% attrition. Baseline-outcome correlations were also estimated. These, however, were generally low; only that for the 24-week MAS score was sufficiently large to enable a useful reduction in target sample size. For both the HRSD and MAS, average scores were low throughout, which may bring into question the appropriateness of these as primary outcome measures. The estimated median time to relapse in TAU was 8.5 weeks, which is somewhat lower than previous research on a similar population (14 weeksReference Sajatovic, Gyulai, Calabrese, Thompson, Wilson and White38). Using a conservative median time to relapse of 18 weeks, if participants were followed up for relapse for 52 weeks (SCID-LIFE data with data from case notes where needed), using a Cox regression model (with 5% significance level), 320 participants would be needed to achieve 90% power to detect a hazard ratio of 2/3.
Limitations
There are a number of limitations to the study. First, information on what constituted TAU was limited. Referral route, medication use and information on previous psychological therapy were collected to help define this; however, it would be useful to collect information regarding current level of care for a future trial. Second, it was not feasible to employ a blinded research assistant to carry out the assessment and follow-up. Third, data from people who had few or no sessions of RfT-OA were lacking for the qualitative interviews. Finally, this was a relatively small study, conducted in North-West England, with a predominantly White British sample. A definitive trial would need to recruit a more geographically and ethnically diverse sample to support generalisability of findings.
Lessons for a future trial
There was substantially more interest from the self-referral recruitment route compared with the clinical route. A well-resourced research team would be poised to form stronger links with clinical teams, generating further interest and increasing the recruitment rate through both routes. A number of strategies have been identified from recent researchReference Robinson, Appelbe, Dodd, Flowers, Johnson and Jones39 to enhance completion rates for self-report measures, including providing detailed explanations regarding the importance of data completeness in recruitment materials, collecting multiple contact details and reminders via different methods (e.g. telephone, text, post, email). Preference for a face-to-face interview for follow-up was highlighted in the qualitative interviews, therefore face to face, video or audio call could be offered to support completion of self-report measures. Paying participants for completing the measures could further enhance retention and is considered good practice with the National Institute for Health Research. Additionally, the observer measures may have been affected by bias. A definitive trial would be fully costed to employ independent, blind assessors to avoid any risks of bias. Finally, two participants did not attend six sessions of therapy: one person became unwell and did not stabilise before the end of the 6-month therapy window, and one person dropped out because of personal reasons. In a future trial, the option of extending the therapy window will be considered with a view to re-engaging participants who disengage with therapy during the trial.
Despite these limitations, the trial was successful in demonstrating the feasibility and acceptability of recruitment, retention and intervention processes. The majority of participants started therapy when offered and adhered to the intervention, reporting positive benefits. The assessment measures provide evidence for a signal for effectiveness on a range of outcomes, including mood symptoms, time to relapse and functioning. The impact on time to relapse, in particular, suggests that RfT-OA may be linked to reduced service costs; however, this would require formal evaluation in a definitive trial. A definitive trial is now warranted to provide a robust estimate of the clinical and cost effectiveness of RfT-OA, and to provide an important step for a group of individuals who, at present, do not have access to evidence-based psychological care.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2022.582
Data availability
The data that support the findings of this study are available from the corresponding author, E.T., upon reasonable request. The transcripts from the qualitative interviews cannot be published or made available if requested, to protect the anonymity of the participants who took part.
Acknowledgements
The authors would like to thank all of the individuals who took part in the study, the service user reference group and the trial steering committee.
Author contributions
E.T., S.H.J., F.L. and C.S. designed the study. E.T., D.D. and B.H. collected, inputted and analysed data. C.S. provided statistical expertise and supervision. E.T. wrote the manuscript. S.H.J., F.L., C.S., S.J. and C.D. proofed and edited the manuscript. S.H.J., C.D. and S.J. provided expertise for the intervention (RfT-OA) described in the manuscript.
Funding
This study was funded by the National Institute for Health Research through a Doctoral Research Fellowship programme grant (number DRF-2014-07-094) awarded to E.T.
Declaration of interest
None.










eLetters
No eLetters have been published for this article.