Betaine is a non-toxic and chemically stable compound that is extensively distributed in nature(Reference Zhao, He and Wu1). It was initially identified in the juice of sugar beets (B. Vulgaris) and has subsequently been observed in other organisms(Reference Lee, An and Nguyen2). Foods containing betaine include wheat products, spinach, beets and liver, among others(Reference Zeisel, Mar and Howe3). However, the precise amount of dietary betaine depends both on the food source and cooking method(Reference Zeisel, Mar and Howe3). Dietary betaine intake influences betaine content in kidneys, liver and brain which seem to be the primary destinations of ingested betaine(Reference Craig4). Intakes of 9–15 g of betaine appear to be safe in humans(Reference Craig4). Besides dietary intake, betaine can be made from choline in the human body(Reference Ashtary-Larky, Bagheri and Ghanavati5). Previous studies indicate that betaine supplementation may improve cardiovascular risk and inflammatory status(Reference Ashtary-Larky, Bagheri and Ghanavati5,Reference Wu, He and Bu6) .
While ‘betaine’ technically refers to a class of related molecules, the term is most commonly used to describe a glycine molecule with three additional methyl groups, termed trimethylglycine(Reference Craig4). Reports have shown that both choline and betaine supplements can combat obesity in animals, including rats(Reference Ge, Yu and Xu7), pigs(Reference Daily, Hongu and Mynatt8,Reference Virtanen and Campbell9) and chickens(Reference Simon10). Moreover, human studies have reported that higher betaine concentration is related to lower BMI, body fat percentage (BFP) and waist circumference(Reference Lever, George and Atkinson11–Reference Chen, Liu and Liu13). Recently, a meta-analysis of six randomised controlled trials (RCT) by Gao et al. has shown that betaine supplementation significantly reduced fat mass (FM) and BFP without affecting body mass (BM) and BMI(Reference Gao, Zhang and Guo14). However, the previous meta-analysis(Reference Gao, Zhang and Guo14) failed to include two RCT that did not show any significant changes in FM and/or BFP following betaine supplementation(Reference Del Favero, Roschel and Artioli15,Reference Rajdl, Racek and Trefil16) . Moreover, a recently published study failed to show any beneficial effects of betaine supplementation on BFP(Reference Moro, Badiali and Fabbri17). Therefore, the previous meta-analytic findings did not fully reflect the documented effects of betaine supplementation on body composition. As a result, there is no clear consensus regarding the overall utility of betaine supplementation for body composition changes.
Dietary changes have typically been considered the primary component for improving body composition in obesity management(Reference Ashtary-Larky, Ghanavati and Lamuchi-Deli18). Numerous studies have shown that alteration in dietary intake (carbohydrate, protein, fat and total energy) can result in body composition alterations(Reference Ashtary Larky, Bagheri and Abbasnezhad19–Reference Layman, Evans and Erickson22). Since the existing literature offers contradictory findings of the effects of betaine supplementation on body composition, it seems necessary to evaluate whether differences in dietary intake concurrent with betaine supplementation could have influenced these findings. Therefore, we aimed to conduct a systematic review and meta-analysis of the pooled data from RCT in adult humans to compare the efficacy of betaine supplementation for altering body composition indices (BM, BMI, FM, BFP and fat-free mass (FFM)) and influencing dietary intake (carbohydrate, protein, fat and total energy).
Experimental methods
The present systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines(Reference Moher, Liberati and Tetzlaff23).
Data sources and search strategies
A comprehensive literature search of five databases, including PubMed, the Cochrane Library, Web of Science, Embase and SCOPUS, was performed using the related terms ‘betaine’, ‘betaine supplementation’ and ‘trimethylglycine’, in combination with the keywords ‘body composition’, ‘anthropometry’, ‘weight’, ‘body mass’, ‘fat mass’, ‘body fat percentage’, ‘waist circumference’, ‘hip circumference’, ‘fat-free mass’, ‘lean body mass’, ‘lean mass’, ‘body mass index’, ‘BMI’, ‘weight loss’, ‘fat loss’, dietary intake’, ‘intake’, ‘diet’, ‘carbohydrate’, ‘fat’, ‘protein’, ‘calorie’, ‘energy’, to identify studies published until 18 December 2020. The process of the search strategy is shown in the flow diagram (Fig. 1).

Fig. 1. Flow diagram of the literature search.
Study selection and eligibility criteria
Two investigators (DAL and SK) selected eligible studies separately by reading the full-text versions of them. All human RCT, either parallel or crossover designs, which reported the effect of betaine supplementation on body composition indices including BM, BMI, FM, BFP, FFM and dietary intake (carbohydrate, protein, fat and total energy) on adults (> 18 years) and published in the English language were considered. The following studies were excluded: (1) RCT with follow-up period < 10 d, (2) studies without a control/placebo arm. When resolution could not be obtained, a third author (RB) was involved by consensus. The participants, intervention, comparators, outcomes and study design criteria are listed in Table 1.
Table 1. Participants, intervention, comparators, outcomes, study design (PICOS) criteria for inclusion of studies

RCT, randomised controlled trials.
Data extraction
The following data were extracted from the full-text versions of eligible studies using a pre-designed abstraction form: first author, year of publication, country, number of participants, study design, the dose of interventions and study duration. In cases of lack of relevant data, the authors were contacted via email to obtain additional information. Two investigators (DAL and SS) carried out the process of data extraction from the original publications independently in order to minimise potential errors. When resolution could not be obtained, a third author (RB) was involved by consensus.
Quality assessment of studies
The quality of studies for assessing the risk of bias was assessed by the Cochrane Collaboration’s tool as previously described(Reference Higgins, Altman and Gøtzsche24). Briefly, nine items were scored, and these items were divided into six domains of bias with three rating categories available for each item: (1) low risk of bias; (2) unclear risk of bias and (3) high risk of bias. The quality of each selected article was assessed by two authors (DAL and WK). When resolution could not be obtained, a third author (OA) was involved by consensus (Table 2).
Table 2. Quality assessment (method: Cochrane collaboration’s tool for assessing risk of bias)

L, low; U, unclear; H, high.
Meta-analysis of data
To analyse the effect size for BM, BMI, FM, BFP, FFM, dietary carbohydrate, protein, fat and total energy, the mean change and its sd were extracted for intervention and control groups, the latter of which served as the comparison group. To calculate the effect size of each study, we used the mean change and sd of the outcome measures from baseline to the end of the intervention in the control and intervention groups(Reference Higgins, Thomas and Chandler25). When the outcome measure was reported as mean and 95 % CI or mean and sem, values were estimated using Review Manager 5·3 software. If the outcome measures were reported in median, range, or 25th–75th percentiles, mean and sd values were estimated using the following formula:
 $$SD = \sqrt {\matrix{
{({{(SD\;pretreatment)}^2} + {{(SD\;posttreatment)}^2}} \hfill \cr
{ - (2R \times SD\;pretreatment \times SD\;posttreatment))} \hfill \cr
} } $$
$$SD = \sqrt {\matrix{
{({{(SD\;pretreatment)}^2} + {{(SD\;posttreatment)}^2}} \hfill \cr
{ - (2R \times SD\;pretreatment \times SD\;posttreatment))} \hfill \cr
} } $$
published by Wan et al., where R = 0.8 was assumed as a correlation coefficient(Reference Wan, Wang and Liu26). If the outcome measures were only reported in figures, we used software (GetData Graph Digitizer) to estimate the value. When an sem or se was reported instead of sd, the sd was calculated based on the following formula: sd = sem × √n (n = sample size in each group).
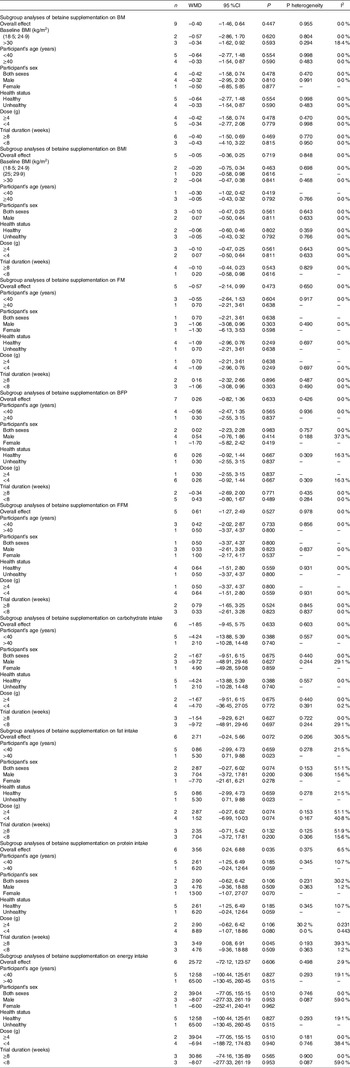
A random effects model was used to calculate weighted mean differences (WMD) with 95 %CI. Between-study heterogeneity was tested by Cochran’s Q test and quantified by I2 statistics. A subgroup analysis based on BMI ((18·5; 24·9 kg/m2), (25; 29·9 kg/m2), or ≥ 30 kg/m2), duration of the study (< 8 or ≥ 8 weeks), the intervention dose (> 4 g or ≤ 4 g/d) and health status (healthy or unhealthy) was conducted to detect potential sources of heterogeneity. Participants with any chronic disease such as diabetes and prediabetes, fatty liver disease, and obesity were considered as unhealthy in health status subgroup(Reference Ashtary-Larky, Kashkooli and Bagheri27,Reference Upadhyay, Farr and Perakakis28) . Moreover, studies which stated their participants as healthy and/or excluded the participants with any diseases were considered as healthy in the subgroup. Sensitivity analysis was conducted by removing each study one by one and recalculating the pooled evaluations. Egger’s regression asymmetry test and visual inspection of funnel plots were performed to detect potential publication bias. Statistical analysis was conducted using STATA, version 11·2 (Stata Corp). The statistically significant value was set at P < 0·05.
Results
Selection and identification of studies
Out of the initial 2575 potentially relevant reports that were obtained by searching the databases, 774 were immediately excluded due to duplication. In addition, we further excluded 1781 studies since they were unrelated to the present meta-analysis (according to our inclusion criteria). From these, 1265 studies did not use betaine supplementation, 482 were conducted on animals, and 34 were review studies. After full-text analysis of the remaining twenty articles, nine studies(Reference Bloomer, Farney and Trepanowski29–Reference Armstrong, Casa and Roti37) were excluded, since they did not evaluate body composition and/or dietary intake following betaine supplementation. Furthermore, one investigation was detected via manual search(Reference Tiihonen, Saarinen and Alhoniemi38) (by searching the references of relevant studies). In total, twelve studies were included in the final analysis(Reference Del Favero, Roschel and Artioli15–Reference Moro, Badiali and Fabbri17,Reference Tiihonen, Saarinen and Alhoniemi38–Reference Grizales, Patti and Lin46) .
Characteristics of studies
According to Cochrane scores, eleven from twelve included studies were classified as high-quality studies (score ≥ 3), and one was classified as low quality (score < 3). The result of the quality assessment is reported in Table 2. Overall, 12 RCT with 13 treatment and 13 control arms were included in the analysis, with a total sample size of 369 participants, out of which 199 participants were in the betaine group/condition and 195 belonged to the control group/condition. The discrepancy between total participants and participants in each group/condition is due to the inclusion of two crossover studies(Reference Lee, Maresh and Kraemer41,Reference Trepanowski, Farney and Mccarthy43) . The mean age of participants in these studies ranged from 21 to 59 years. These studies were published between the years 2002 and 2020. The studies were conducted in Finland(Reference Schwab, Törrönen and Toppinen39,Reference Schwab, Alfthan and Aro42) , USA(Reference Abdelmalek, Sanderson and Angulo40,Reference Lee, Maresh and Kraemer41,Reference Trepanowski, Farney and Mccarthy43–Reference Grizales, Patti and Lin46) , Brazil(Reference Del Favero, Roschel and Artioli15), Japan(Reference Tiihonen, Saarinen and Alhoniemi38), Czech Republic(Reference Rajdl, Racek and Trefil16) and Italy(Reference Moro, Badiali and Fabbri17). A daily dose of betaine ranged from 1·5 to 20 g/d. The trial duration also varied from 2 to 52 weeks. Study participants included patients with prediabetes(Reference Grizales, Patti and Lin46), fatty liver disease(Reference Tiihonen, Saarinen and Alhoniemi38,Reference Abdelmalek, Sanderson and Angulo40) , obesity(Reference Schwab, Törrönen and Toppinen39) and healthy individuals(Reference Del Favero, Roschel and Artioli15–Reference Moro, Badiali and Fabbri17,Reference Lee, Maresh and Kraemer41–Reference Cholewa, Hudson and Cicholski45) . The characteristics of twelve studies eligible for inclusion in the present meta-analysis are shown in Table 3.
Table 3. The characteristics of the included trials

BM, body mass; BFP, body fat percentage; FM, fat mass; FFM, fat-free mass; ND, non-defined; ↔, unchanged, ↓, decreased; ↑, increased.
Meta-analysis of data
Effects of betaine supplementation on body mass and BMI
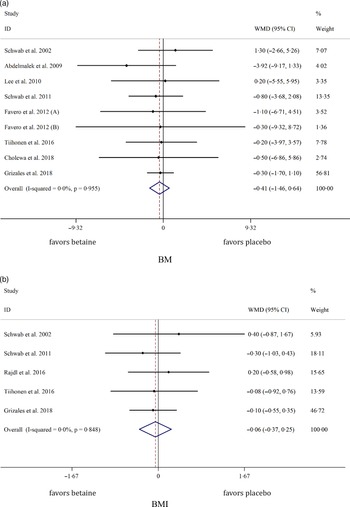
Pooled analysis of nine and five effect sizes indicated that the participants assigned to betaine supplementation did not change BM (WMD: −0·40 kg, 95 % CI −1·46, 0·64, I2 = 0·0 %, P = 0·447) or BMI (WMD: −0·05 kg/m2, 95 % CI −0·36, 0·25, I2 = 0·0 %, P = 0·719) in comparison with placebo (Fig. 2(a): BM and Fig. 2(b): BMI). Subgroup analysis showed that betaine supplementation in different doses (≥ 4 or < 4 g), participant’s age (< 40 and ≥ 40 years), sex (male, female and both sexes) and trial duration (≥ 8 or < 8 weeks) did not have significant effects on BM or BMI (Table 4).

Fig. 2. Forest plot of the random effects meta-analysis of the effect of betaine supplementation on (a) BM and (b) BMI. BM, body mass. WMD, weighted mean difference.
Table 4. Subgroup analysis of betaine supplementation on body composition

WMD, weighted mean differences; BM, body mass; BFP, body fat percentage; FM, fat mass; FFM, fat-free mass.
Effects of betaine supplementation on body fat percentage and fat mass
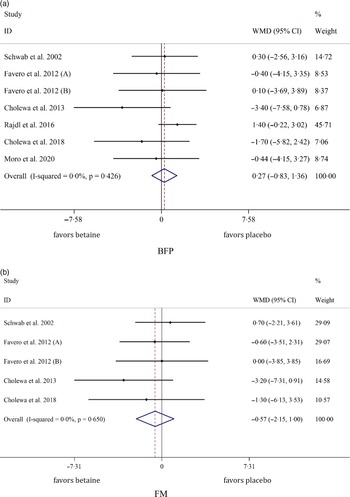
Overall, seven arms indicated the effects of betaine supplementation on BFP. Our results showed that betaine supplementation did not affect BFP (WMD: 0·26 %, 95 % CI −0·82, 1·36, I2 = 0·0 %, P = 0·633) in comparison with control (Fig. 3(a)). Moreover, from five arm assessed, betaine supplementation failed to change FM (WMD: −0·57 kg, 95 % CI −2·14, 0·99, I2 = 0·0 %, P = 0·473) (Fig. 3(b)). Subgroup analysis showed that betaine supplementation in different doses (≥ 4 or < 4 g), participant’s age (< 40 and ≥ 40 years), sex (male, female and both sexes), trial duration (≥ 8 or < 8 weeks) and health status (healthy or unhealthy) did not have significant effects on BFP or FM (Table 4).

Fig. 3. Forest plot of the random effects meta-analysis of the effect of betaine supplementation on (a) BFP and (b) FM. BFP, body fat percentage; FM, fat mass. WMD, weighted mean difference.
Effects of betaine supplementation on fat-free mass
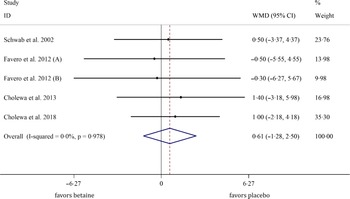
In total, five studies evaluated the effects of betaine supplementation on FMM. Forest plots summarising the efficacy of betaine supplementation on FFM are shown in Fig. 4. The individuals supplemented with betaine were shown insignificant changes of FFM ((WMD: 0·61 kg, 95 % CI −1·27, 2·49, I2 = 0·0 %, P = 0·527), Fig. 4)). Subgroup analysis showed that betaine supplementation in different doses (≥ 4 or < 4 g), participant’s age (< 40 and ≥ 40 years), sex (male, female and both sexes), trial duration (≥ 8 or < 8 weeks) and health status (healthy or unhealthy) did not significantly affect FFM (Table 4).

Fig. 4. Forest plot detailing weighted mean difference and 95 % CI for the effect of betaine supplementation on FFM. FFM, fat-free mass. WMD, weighted mean difference.
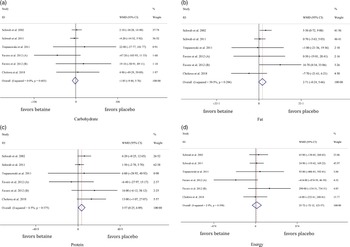
Effects of betaine supplementation on dietary intakes
In total, six studies evaluated dietary intakes following betaine supplementation. There was no significant difference in energy intake between the groups (WMD: 107·61 kJ, 95 % CI –72·12, 123·57, I2 = 2·9 %, P = 0·606) (Fig. 5(d)). Moreover, pooled analysis of six effect sizes demonstrated that dietary protein intake may have marginally increased with betaine supplementation as compared with control ((WMD: 3·56 g, 95 % CI 0·24, 6·88, I2 = 6·5 %, P = 0·035), Fig. 5(c))) without differences for dietary carbohydrate ((WMD: –1·85 g, 95 % CI –9·45, 5·75, I2 = 0·0 %, P = 0·633), Fig. 5(a))) and fat intake ((WMD: 2·71 g, 95 % CI –0·24, 5·66, I2 = 30·5 %, P = 0·072), Fig. 5(b))).

Fig. 5. Forest plot of the random effects meta-analysis of the effect of betaine supplementation on (a) dietary carbohydrate intake, (b) dietary fat intake, (c) dietary protein intake and (d) dietary energy intake. WMD, weighted mean difference.
Sensitivity analysis
The sensitivity analysis showed that results for each variable were not substantially changed after excluding each study individually.
Publication bias
The results of Egger’s test revealed that with the exception of BFP (P = 0·009), there was no publication bias for outcome variables (BM, P = 0·809; BMI, P = 0·302; FM, P = 0·351; FFM, P = 0·161; carbohydrate, P = 0·733; fat, P = 0·400; protein, P = 0·657; and energy, P = 0·687). Funnel plots for outcome variables are presented in Supplementary file 1. Due to significant publication bias for BFP, we conducted trim and fill analysis, and the results of the analysis showed that with the publication of seven new studies, the results of the publication bias would be non-significant. However, the estimated effects of betaine supplementation on BFP did not significantly change (WMD: 0·26 %, 95 %CI −0·82, 1·36, P = 0·633).
Discussion
In this meta-analysis, we evaluated the effects of betaine supplementation on body composition indices. According to the results derived from this study, betaine supplementation failed to affect body composition indices, including BM, BMI, FM, BFP and FFM. Moreover, our findings showed that those who supplemented with betaine potentially had a slightly increased dietary protein intake, although the magnitude of the difference is negligible. Furthermore, subgroup analyses based on the duration of interventions (≤ 8 and > 8 weeks), participant’s age (< 40 and ≥ 40 years), sex (male, female and both sexes), betaine dose (< 4 and ≥ 4 g/d), baseline BMI ((18·5; 24·9 kg/m2), (25; 29·9 kg/m2) or ≥ 30 kg/m2) and health status (healthy or unhealthy) did not reveal any significant differences.
Prior meta-analytic work of six RCT by Gao et al. evaluated the effects of betaine supplementation on body composition(Reference Gao, Zhang and Guo14). They have shown that betaine supplementation significantly reduced FM and BFP without affecting BM and BMI(Reference Gao, Zhang and Guo14). The present findings exhibit some discrepancies with the results of the study by Gao et al., who provided new knowledge on several relevant topics. First, Gao et al. included only four studies for assessing the effects of betaine supplementation on FM and BFP while we included five and seven trials, respectively. The lower number of included studies in the previous meta-analysis resulted in one study(Reference Cholewa, Wyszczelska-Rokiel and Glowacki44) having relatively high weight in the analysis (68·83 % and 68·44 % for FM and BFP, respectively), which might have influenced the trend of the pooled effects of betaine supplementation on body fat. Second, some aspects of the meta-analytical methods may have differed between investigations. For example, Gao et al. reported a much narrower 95 % CI for the study of Cholewa et al. (Reference Cholewa, Wyszczelska-Rokiel and Glowacki44) in terms of FM reduction (–4·96 kg, –1·44 kg) as compared with the CI calculated in the present investigation (–7·31 kg, 0·91 kg). This is notable as this was the same investigation that had a very high weight (68·83 %) in the previous meta-analysis study. Third, Gao et al. (Reference Gao, Zhang and Guo14) did not evaluate the influence of betaine supplementation on FFM, while we showed that betaine supplementation was unable to induce any significant changes in FFM. Fourth, due to the limited trials in the previous study, subgroup analysis was not performed. However, we showed the effects of betaine supplementation in different subgroups based on duration, dosage and baseline BMI. Fifth, we analysed dietary intake to determine if there were any significant changes following betaine supplementation. Therefore, the inclusion of seven additional studies, increasing the sample size by 89 % in the current analysis, has a substantial influence on the available evidence. In contrast with the previous meta-analysis(Reference Gao, Zhang and Guo14), our findings suggest that betaine supplementation does not improve body composition indices.
In healthy adults, serum betaine concentration is normally between 20 and 60 μmol/l(Reference Lever, Sizeland and Bason47). From all included studies, the two studies by Cholewa et al. (Reference Cholewa, Wyszczelska-Rokiel and Glowacki44,Reference Cholewa, Hudson and Cicholski45) reported significant body fat-lowering effects of betaine supplementation; however, betaine concentration was not measured in these investigations. Nevertheless, based on the other included studies reporting serum concentration of betaine(Reference Rajdl, Racek and Trefil16,Reference Tiihonen, Saarinen and Alhoniemi38,Reference Schwab, Törrönen and Toppinen39,Reference Schwab, Alfthan and Aro42,Reference Grizales, Patti and Lin46) , it seems that all participants had normal or nearly normal betaine concentration. Moreover, these investigations failed to show any significant difference in body composition indices(Reference Rajdl, Racek and Trefil16,Reference Tiihonen, Saarinen and Alhoniemi38,Reference Schwab, Törrönen and Toppinen39,Reference Schwab, Alfthan and Aro42,Reference Grizales, Patti and Lin46) . These results suggest that at least when betaine concentration is normal or near-normal, betaine supplementation may not affect body composition indices. To reveal the extent to which the effects of betaine supplementation are dependent on baseline concentration, studies could be conducted in individuals with varying serum concentration of betaine.
Betaine is a commonly used additive in the animal’s diet. Some studies showed that betaine is effective in increasing lean mass in pigs(Reference Campbell, Morley and Zabaras-Krick48–Reference Cromwell, Lindemann and Randolph50) and chickens(Reference Saunderson and Mackinlay51). The possible mechanism for increasing lean mass in animals is unclear. However, animal studies showed that betaine supplementation may act as a methyl donor in methionine-deficient diets and may result in growth rates comparable to animals fed diets with adequate methionine(Reference Campbell, Cadogan and Morley52–Reference Lawrence, Schinckel and Adeola54). For example, a study by Saunderson et al. demonstrated reductions in FM and increased protein content of chicken breast following betaine supplementation(Reference Saunderson and Mackinlay51). However, there is no evidence that betaine supplementation can improve FFM in humans. All studies included in our analysis reported a non-significant change of FFM following betaine supplementation(Reference Del Favero, Roschel and Artioli15,Reference Schwab, Törrönen and Toppinen39,Reference Cholewa, Wyszczelska-Rokiel and Glowacki44,Reference Cholewa, Hudson and Cicholski45) . Therefore, betaine supplementation appears to reduce FM and increase FFM in animals, but the results from our analysis do not support the efficacy of betaine supplementation to alter these indices in humans.
Dietary intake changes are the most important factor affecting the rate of BM and FM alterations(Reference Champagne, Broyles and Moran55). In agreement with the ‘calories in, calories out’ theory, the unchanged energy intake following betaine supplementation corresponded to no changes in BM and BMI. Although protein intake significantly increased following betaine supplementation, the magnitude was very small and not clinically significant. Overall, based on the current evidence, betaine supplementation is not an effective supplement for BM loss(Reference Schwab, Törrönen and Toppinen39,Reference Abdelmalek, Sanderson and Angulo40,Reference Grizales, Patti and Lin46) .
Only three of the included studies were conducted in individuals with overweight and obesity. Further long-term interventional studies, especially in populations with obesity, may be needed to allow for additional evaluation of the influence of betaine supplementation on body composition indices. As mentioned, examining the potential influence of betaine supplementation in those with suboptimal concentration may reveal differential effects as compared with those with normal concentrations.
Betaine supplementation may not be effective for improving BM, BMI, FM, FFM and BFP. Moreover, our findings showed that dietary intake did not meaningfully change following betaine supplementation. Additional longer-term and high-quality studies are needed to further evaluate and confirm these findings.
Acknowledgements
None.
No funding received.
Conceptualisation: R. B. and D. A. L.; methodology: D. A. L., S. S., S. K., W. K. and R. B.; software: O. A.; writing – original draft preparation: R. B. and D. A. L.; writing – review and editing: R. B., D. A. L., G. M. T. and A. W. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.