Given its well-established beneficial effects in terms of preventing neural tube defects (NTD)(Reference Berry, Li and Erickson1,Reference Czeizel and Dudas2) , peri-conceptional folic acid (FA) is universally recommended for women of reproductive age, including women in early pregnancy and those planning to become pregnant. The intake of supplements that contain FA is correlated with a reduced risk for orofacial cleft(Reference Li, Chao and Li3), limb deficiency(Reference Shaw, Nelson and Carmichael4,Reference Liu, Li and Ye5) , imperforate anus(Reference Myers, Li and Correa-Villasenor6) and other defects(Reference Shaw, Nelson and Carmichael4). Concerns over preventive effects against other birth defects are emerging. Peri-conceptional multi-vitamin use was associated with a 60 % reduction in the risk for non-syndromic omphalocele(Reference Botto, Mulinare and Erickson7) in a case–control study of 3100 infants born in 1996–1980 in the USA. A modest decrease (21 %) in the birth prevalence of omphalocele in 1999–2000 (post-FA fortification) compared with 1995–1996 (pre-fortification) was observed in a population-based study of nine states in the USA(Reference Canfield, Collins and Botto8). Furthermore, a gene study found that SNP related to folate and vitamin B12 were associated with omphalocele among 930 births (169 non-aneuploid omphalocele cases and 761 unaffected controls) in New York State in 1998–2005(Reference Mills, Carter and Kay9), although there was no report for prevention effect of FA only on omphalocele.
Omphalocele and gastroschisis are the most common fetal congenital abdominal wall defects (AWD), which refer to an opening in the abdomen through which various abdominal organs can protrude. Free-floating bowel loops and other viscera herniate outside the body in gastroschisis, while omphalocele is a membrane-covered midline defect(Reference Gamba and Midrio10,Reference Pakdaman, Woodward and Kennedy11) . AWD are life-threatening malformations associated with significant morbidity, mortality and prolonged hospitalisation. Omphalocele is usually complicated with intra-uterine growth retardation, pre-mature, respiratory insufficiency, gastroesophageal reflux and feeding difficulty(Reference Gamba and Midrio10).
The prevalence of AWD varies worldwide. The prevalence of gastroschisis and omphalocele ranges from 1–4 to 2–3 per 10 000 live births and has shown an increasing trend in recent years(Reference Oluwafemi, Benjamin and Navarro Sanchez12). The prevalence tends to be higher in North America and Europe than in Asia. The prevalence of gastroschisis and omphalocele was 1·79 and 1·60 per 10 000 live births in a large German county in 2010–2015(Reference Wittekindt, Schloesser and Doberschuetz13), 3·34 and 1·55 per 10 000 live births in the USA in 1999–2007(Reference Kirby14) and 1·76 and 2·18 per 10 000 births in France in 1979–1998, respectively(Reference Stoll, Alembik and Dott15). It was 0·2–1·2 and 1·0–1·7, respectively, in the Czech Republic in 2000–2008(Reference Sipek, Gregor and Horacek16). The overall prevalence of gastroschisis and AWD was 0·50 and 1·65 per 10 000 births, respectively, in Taiwan during 1998–2013(Reference Chen, Chen and Chen17). The increased incidence is mainly due to the widespread use of prenatal ultrasound(Reference Gamba and Midrio10). The prevalence at birth of gastroschisis increased significantly from 8 to 35 (average = 10·7) per 10 000 live-born and stillborn infants, and the prevalence of omphalocele ranged from 8 to 11, from 1989 to 2015 in Greenland(Reference Bugge, Drachmann and Kern18).
The aetiology of AWD is generally thought to be multi-factorial and remains largely unknown. Gastroschisis and omphalocele generally have different risk factors. Smoking, pre-gestational or gestational diabetes, the use of medication to treat depression and younger maternal age are significantly associated with gastroschisis(Reference D’Antonio, Virgone and Rizzo19–Reference Perry, Mulcahy and DeFranco21). In contrast, obesity, family history, genetic mutation and chromosomal abnormalities are risk factors for omphalocele(Reference Sazhin, Zolotukhin and Seliverstov22). Omphalocele can be secondary to an NTD as well. Taking vitamins that contain FA, both FA and vitamin B12, around the time of conception and consuming food fortified with FA are beneficial for preventing omphalocele. Genetic factors are aetiologically important in omphalocele as well: variants of the transcobalamin receptor gene, transcobalamin II, betaine-homocysteine S-methyltransferase(Reference Mills, Carter and Kay9) and methylenetetrahydrofolate reductase(Reference Mills, Druschel and Pangilinan23) are significantly associated with omphalocele. Vitamin insufficiency, including folate or cobalamin insufficiency, may impair methylation reactions, contributing to omphalocele.
To date, there is little evidence of the protective effect of FA alone on AWD from prospective cohort studies or randomised controlled trials. Using data from a large birth cohort study in a public health campaign from 1993 to 1996 in China established to evaluate the effects of FA supplementation alone on the prevention of external structural birth defects, we examine the potential preventive effects of FA supplementation on omphalocele, gastroschisis and total AWD in China.
Methods
The methods of the original study have been described previously(Reference Berry, Li and Erickson1,Reference Liu, Li and Ye5,Reference Li, Ye and Zhang24) . Briefly, in 1993, the Chinese Ministry of Health conducted a public health campaign to prevent NTD in twenty-one counties in southern China (Zhejiang and Jiangsu) and northern China (Hebei). A well-organised pregnancy-monitoring system was established to collect principal records of prenatal care and demographic information. All women who were preparing for marriage or who became pregnant in the project counties were registered. The enrolled women were advised to take a tablet 0·4 mg FA every day, starting at the time of registration with the pregnancy-monitoring system and continuing until completion of the first trimester of pregnancy. If women consented to take FA, the tablets were distributed at the time of registration. At the end of each month, health workers recorded the dates of all menstrual periods and how many tablets remained in each bottle (if taking tablets). All births at 20 complete gestational weeks, including live births, stillbirths and pregnancy terminations, and all structural birth defects regardless of gestational week were recorded. The original cohort included 247 831 women who registered with the pregnancy-monitoring system between October 1993 and September 1995 and who delivered by 31 December 1996.
Definition of folic acid use
The classification and patterns of FA consumption were the same as previously reported(Reference Berry, Li and Erickson1). Women who took FA tablets at any time from the registration period until the end of the first trimester of pregnancy were classified as FA users. Women who did not agree to take FA or who were registered during the second trimester of pregnancy (i.e. did not have the opportunity to start taking FA by the end of the first trimester) were considered non-users. Three patterns of FA exposure were established according to the dates women started and stopped taking FA: (1) peri-conception: initiation was before the last menstrual period and termination was within the first trimester, (2) pre-conception: initiation and termination were before the last menstrual period and (3) post-conception: initiation was after last menstrual period and termination was within the first trimester. Compliance was calculated as actual consumed tablets divided by the assigned tablets.
Identification of congenital abdominal wall defects
Congenital AWD were identified through the Birth Defects Surveillance System for the Collaborative Project-China as previously reported(Reference Berry, Li and Erickson1,Reference Li, Moore and Li25) . This system, which was established in January 1993, collects detailed data on infants and fetuses with external structural birth defects. Live-born infants with birth defects were included in the surveillance system if they had a gestational age of at least 20 weeks and had a birth defect that was diagnosed by 6 weeks of age. Photographs of every infant born with a suspected birth defect were recorded and recognised. We also collected information on all pregnancies, even those with gestations of <20 weeks that were electively terminated after the prenatal diagnosis of any birth defect. Three paediatricians, who were unaware of the women’s tablet-taking status, independently reviewed the reports and photographs and assigned diagnostic codes, and a clinical geneticist validated the diagnoses. Congenital AWD were diagnosed according to International Classification of Diseases (ICD)-9-CM code 756.7, which includes gastroschisis, omphalocele and other congenital anomalies of the abdominal wall but excludes umbilical hernia (551–553 with 1) (http://icd9cm.chrisendres.com/index.php?action=child&recordid=7754).
The project was approved by the institutional review boards of the US Centers for Disease Control and Prevention and Peking University Health Science Center. All women who took tablets provided oral informed consent.
Statistical analysis
The sample size was calculated using PASS (v. 20.0.3; NCSS). The main effect of FA supplementation on AWD was estimated. The exposure group was treated with FA, and the control group was not treated with FA. According to previous reports from the UK, the OR of the exposure group relative to the control group is 0·3(Reference Paranjothy, Broughton and Evans26) and it is estimated that the incidence of fetal AWD in the control group is 6 per 10 000 births. If α = 0·05 (bilateral), β = 0·10, according to case:control = 1:1, the sample size of the exposure and control groups is 53 780 cases. A total of 247 831 participants were investigated in our study, which satisfied the statistical requirements. Our study had 99·7 % power (α = 0·15) to detect a decrease of 10 % over the unexposed rate of 2 per 10 000 for fetal gastroschisis.
We compared demographic characteristics including age, BMI, ethnicity, education, occupation and parity between the groups of women who had and had not taken FA. We compared means using t tests and distributions of category using χ 2 tests. We calculated the rate of AWD, omphalocele and gastroschisis (the number of cases per 10 000 pregnancies of at least 20 weeks’ gestation) according to the patterns of FA intake. Risk ratios were estimated through logistic regression, which adjusted for the main potential confounding variables, including maternal age at pregnancy and occupation. All data were analysed using SPSS (v. 20.0; SPSS Inc.).
Results
Table 1 shows select characteristics of the 247 831 participants according to peri-conceptional use of FA. A total of 18 591 (58·2 %) women in the north and 111 151 (51·7 %) in the south took FA. Women who did not take FA supplements were more likely to be multiparous, be older, be a farmer, have a higher BMI and have lower educational levels.
Table 1. Maternal characteristics by folic acid (FA) supplementation, China, 1993–1996*
(Numbers and percentages)

* Values for some characteristics may not be equal to total numbers in each group because of missing values.
A total of 150 cases of AWD were identified in the current study: twenty-six cases in the north and 124 cases in the south. There were forty-one cases of omphalocele and forty-six cases of gastroschisis, which accounted for 58 % of the AWD. Other AWD, including prune belly syndrome (ICD-9: 756.72) and other and unspecified anomalies of the abdominal wall (ICD-9: 756.79), accounted for 42 % of the total AWD in our study. The prevalence of total AWD was 4·30 per 10 000 births among women who took FA compared with 13·46 per 10 000 births among those who did not take FA in northern China and 6·28 and 5·18 per 10 000 births, respectively, in southern China. The prevalence of omphalocele was 0·54 per 10 000 births among women who took FA compared with 3·74 per 10 000 births among those who did not take FA in northern China and 1·79 and 1·44 per 10 000 births, respectively, in southern China. Significant differences were observed according to FA supplementation status in omphalocele and total AWD in northern China (Table 2).
Table 2. Prevalence of congenital abdominal wall defects (AWD) by folic acid (FA) supplementation in China, per 10 000 births
(Numbers and prevalences; risk ratios (RR) and 95 % confidence intervals)
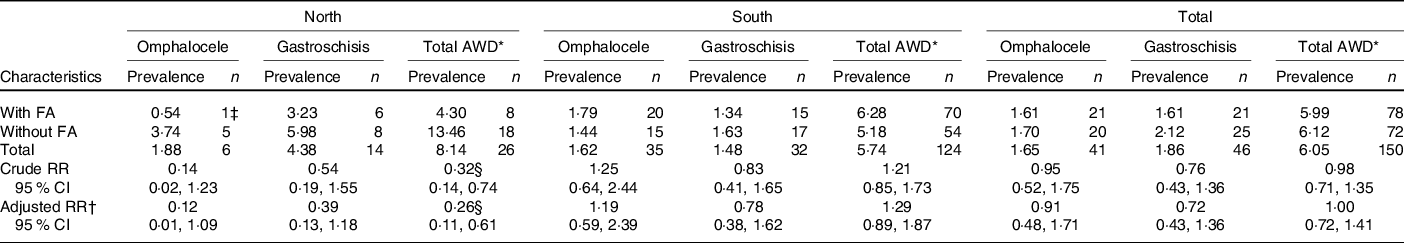
* Total AWD included omphalocele, gastroschisis, prune belly syndrome, other and unspecified anomalies of the abdominal wall.
† Adjusted by age at pregnancy, BMI, parity, ethnicity, education, occupation.
‡ This case was complicated defects: omphalocele, and spina bifida and bladder exstrophy.
§ P < 0·05.
Among five cases of omphalocele in northern China, one case of omphalocele was complicated with an NTD and bladder exstrophy, while among thirty-five cases of omphalocele in southern China, five cases of omphalocele were complicated with NTD. In both areas, gastroschisis occurred in isolation.
FA supplementation significantly prevented total AWD in northern China in univariate (risk ratio 0·32, 95 % CI 0·14, 0·74) and multi-variate (risk ratio 0·26, 95 % CI 0·11, 0·61) analyses (Table 2). No preventive effect of FA on AWD was observed in southern China.
No differences in the prevalence of AWD and subtypes were observed according to compliance with FA use (Table 3).
Table 3. Compliance of folic acid use and risk for congenital abdominal wall defects (AWD) in China, per 10 000 births
(Numbers and prevalences)
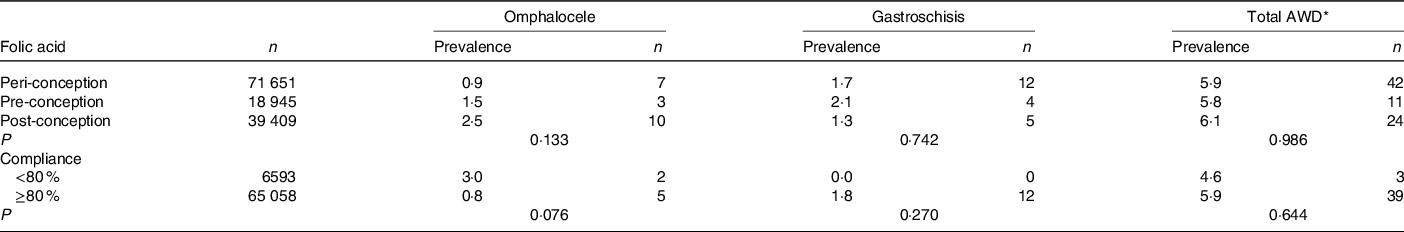
* Total AWD included omphalocele, gastroschisis, prune belly syndrome, other and unspecified anomalies of the abdominal wall.
Discussion
In this large and non-randomised intervention study, we observed a decrease in the risk for fetal AWD, especially omphalocele, among pregnant women who took FA supplements compared with those who did not in northern China. Our study was conducted in a period when there was no massive FA supplementation or food fortification among women of childbearing age in China, few women consumed multi-vitamins(Reference Li, Ye and Zhang24), and the only intervention was FA supplementation alone (0·4 mg FA per tablet). The current study, the largest intervention study to have explored the use of FA in relation to AWD, adds evidence of the preventive effect of FA supplementation alone on fetal congenital AWD among a population with low folate.
Omphalocele is a feature of many genetic syndromes, including trisomy and Beckwith–Wiedemann syndrome. Individuals with omphalocele frequently have multiple birth defects, such as cloacal/bladder exstrophy, imperforate anus and spina bifida (the four symptoms together are also called OEIS) or congenital heart defect(Reference Botto, Mulinare and Erickson7,Reference Stoll, Alembik and Dott27) . Gastroschisis occurs among non-syndromic individuals and in isolation(Reference Oluwafemi, Benjamin and Navarro Sanchez12). Other rarer AWD are pentalogy of Cantrell, bladder and cloacal exstrophy, imperforate anus, spina bifida complex, prune belly syndrome and body stalk anomaly(Reference Prefumo and Izzi28). In the current study, several cases of omphalocele were associated with spina bifida and bladder exstrophy, whereas gastroschisis occurred in isolation.
Omphalocele and gastroschisis are the most frequent congenital AWD. Our study revealed a prevalence of 1·65/10 000, 1·86/10 000 and 6·05/10 000 for omphalocele, gastroschisis and total AWD, respectively, in China. The average prevalence in this study was lower than the global prevalence, which is approximately 2–2·5 in 10 000 newborns for omphalocele and 2–6 in 10 000 newborns for gastroschisis. However, significant differences were observed according to FA supplementation status in omphalocele and all AWD in northern China. The prevalence of omphalocele and total AWD was significantly higher in women who did not take FA than women who did in northern China. We did not observe this effect in southern China. A study from Norway also revealed that FA supplementation was associated with a reduced risk for AWD(Reference Gildestad, Bjørge and Haaland29). The most significant characteristic of women in northern and southern China was a low folate concentration due to various factors. Women in the north had less than half the folate concentration of women in the south; as reported in 2003, erythrocyte folate concentrations were 440·0 nmol/l in the north and 910·4 nmol/l in the south(Reference Ren, Zhang and Hao30).
Although the causes of AWD and the mechanisms underlying them are largely unknown, multiple genetic and environmental factors are probably involved. Mothers of children with AWD often take more medication during pregnancy than mothers of controls(Reference Stoll, Alembik and Dott15). As a midline defect, omphalocele might share common aetiological pathways with NTD and other abnormalities. For example, they are both connected with disrupted production, transport or metabolism of vitamin B12, which was correlated with folate metabolism(Reference Gardiki-Kouidou and Seller31). A previous study showed that peri-conceptional use of multi-vitamin supplements was associated with a 60 % reduction in non-syndromic omphalocele (OR 0·4, 95 % CI 0·2, 1·0) in the USA(Reference Botto, Mulinare and Erickson7), although the results could not suggest that FA alone is effective. The current study included the largest population for observation of the effects of FA alone on omphalocele and AWD in the world.
The present study had several strengths. First, based on a cohort of about 250 000 births, the current study is probably the largest population-based cohort study of its kind and enabled us to explore risk factors for less common birth defects such as AWD. Second, it was based on a well-organised population-based monitoring system that used quality control to ensure data quality and included all births at twenty complete gestational weeks, including live births, stillbirths and pregnancy terminations, and all external structural defects regardless of gestational week(Reference Berry, Li and Erickson1), which provided estimates closer to the actual birth prevalence of these birth defects(32). Third, the only intervention in this study was FA supplementation, and thus the study reflects the pure and single effect of FA alone. The dose of FA used in our study was 0·4 mg. Therefore, we would identify effects associated with this dose. At that time, few women in the study area could afford multiple vitamins and there is few market supply in the multiple vitamins, and the period we observed was before the national FA supplementation programme, which could best reflect the true effect of natural folate status of the population.
However, a couple of limitations should also be noted. First, we did not collect information on the diets and nutrient intake of the women, which may have influenced the results. However, the majority of participants were of Han ethnicity and were relatively homogeneous. The dietary intake of the two regions was different, as reflected in the folate concentrations. The erythrocyte folate concentration in the north was half that in the south(Reference Ren, Zhang and Hao30). We analysed the data by region, but this would not have changed the overall trends observed in our study. Prenatal vitamins were not a part of routine health care service, nor were multi-vitamin supplements available for purchase at the time of this study. Second, because of the very low prevalence of AWD (2–3 per 10 000 births), although the study was based on a large FA intervention study, the small number of affected participants limited the precision of subgroup analyses and translated into a wide CI that included unity. At the same time, given the limited number of affected cases in our sample, we could not perform separate analyses to exclude infants with AWD together with another syndrome. Third, confounding by factors associated with FA use also remains a possibility. However, adjustment for several demographic factors did not change the results appreciably.
In conclusion, FA supplementation successfully reduces the prevalence of omphalocele and total AWD among women in northern China with low folate.
Acknowledgements
The authors thank the staff members and participating women of the original trial.
This work was supported in part by the National Key Research and Development Program, Ministry of Science and Technology of the People’s Republic of China (grant no. 2016YFC1000501) from Aiguo Ren and Natural Science Foundation of China (no. 81373014 from Z. L. and no. 81202265 from J. L.). The original project was supported by a cooperative agreement between the US Centers for Disease Control and Prevention and Peking University (grant no. U01 DD000293).
J. F. L. analysed the data, drafted the initial manuscript and reviewed and revised the manuscript. Z. L. and R. Y. conceptualised and designed the study, and critically revised the manuscript; A. R., R. Y. and J. M. L. coordinated and supervised data collection, reviewed and revised the manuscript. All authors read, reviewed and approved the final manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.








