No CrossRef data available.
Article contents
Minimal processed infant formula vs. conventional shows comparable protein quality and increased postprandial plasma amino acid kinetics in rats
Published online by Cambridge University Press: 23 November 2023
Abstract
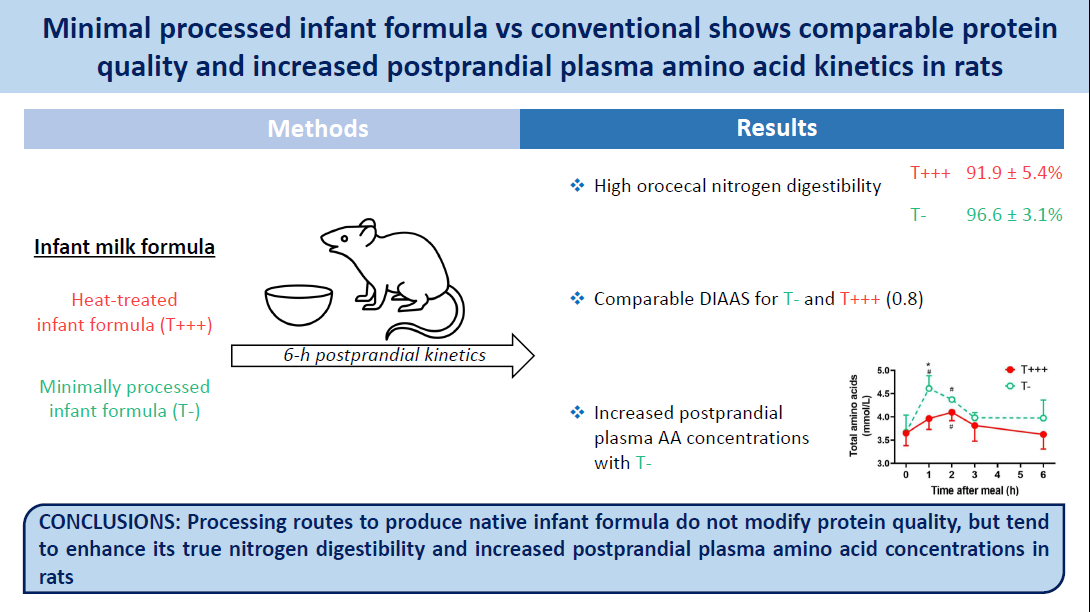
During industrial processing, heat treatments applied to infant formulas may affect protein digestion. Recently, innovative processing routes have been developed to produce minimally heat-processed infant formula. Our objective was to compare the in vivo protein digestion kinetics and protein quality of a minimally processed (T−) and a heat-treated (T+++) infant formula. Sixty-eight male Wistar rats (21 d) were fed with either a diet containing 40 % T− (n 30) or T+++ (n 30), or a milk protein control diet (n 8) during 2 weeks. T− and T+++ rats were then sequentially euthanised 0, 1, 2, 3 or 6 h (n 6/time point) after ingestion of a meal containing their experimental diet. Control rats were euthanised 6 h after ingestion of a protein-free meal to determine nitrogen and amino acid endogenous losses. Nitrogen and amino acid true caecal digestibility was high for both T− and T+++ diets (> 90 %), but a tendency towards higher nitrogen digestibility was observed for the T− diet (96·6 ± 3·1 %) compared with the T+++ diet (91·9 ± 5·4 %, P = 0·0891). This slightly increased digestibility led to a greater increase in total amino acid concentration in plasma after ingestion of the T− diet (P = 0·0010). Comparable protein quality between the two infant formulas was found with a digestible indispensable amino acid score of 0·8. In conclusion, this study showed that minimal processing routes to produce native infant formula do not modify protein quality but tend to enhance its true nitrogen digestibility and increase postprandial plasma amino acid kinetics in rats.
Keywords
- Type
- Research Article
- Information
- Copyright
- © The Author(s), 2023. Published by Cambridge University Press on behalf of The Nutrition Society





