Rheumatoid arthritis (RA) is a chronic autoimmune disease of unknown origin that primarily affects the joints and ultimately leads to their destruction( Reference Huber, Distler and Tarner 1 ). Nowadays, although the aetiopathogenesis of RA is only partially understood, it is known that the involvement of immune cells and their respective proinflammatory mediators is a common hallmark of RA as of all systemic autoimmune disorders( Reference Smith and Haynes 2 ).
Synovial fibroblasts (SF) are unique cells that populate the intimal lining of the synovium( Reference Bottini and Firestein 3 ). In non-affected healthy joints, resident SF are responsible for continuous physiologic remodelling of the matrix, whereas in RA patients SF present an altered phenotype and play a pivotal role in the pathogenesis of RA( Reference Neumann, Lefevre and Zimmermann 4 ). RA-SF attach to the articular cartilage, produce cartilage matrix-degrading enzymes and once cartilage is eroded they invade the underlying bone and activate osteoclast-mediated bone resorption. Various proinflammatory mediators, including IL-6, IL-1β, TNF-α and matrix metalloproteinases (MMP), released from RA-SF are involved in the destruction of both articular bone and cartilage( Reference Feldmann and Maini 5 ). The regulation of these pro-inflammatory mediator gene expressions involves multiple signal transduction pathways including mitogen-activated protein kinases (MAPK), which comprise extracellular signal-regulated kinases (ERK1/2 or p42/p44), c-Jun N-terminal kinases (JNK)1/2/3 and p38 and the NF-κB, which is also activated in RA-SF and appears to be important in perpetuating disease, as well as in mediating synovial inflammation( Reference Pap, Muller-Ladner and Gay 6 ). Particularly, NF-κB plays an important role in MMP induction and also regulates a wide range of genes that contribute to inflammation, such as IL-1β, TNF-α, IL-6, chemokines and microsomal PG E synthase-1 (mPGES-1), an efficient downstream enzyme co-localised and functionally coupled with the inducible enzyme cyclo-oxygenase (COX)-2( Reference McCoy, Wicks and Audoly 7 ). An excessive production of cytokines by RA-SF has been shown to induce proliferation of these cells and facilitate invasion into the adjacent tissues( Reference Garcia-Vicuna, Gomez-Gaviro and Dominguez-Luis 8 ). These lead to the destruction of articular cartilage and subchondral bone, resulting in joint deformity and a great deal of pain in RA patients.
Although new biological therapies have improved the treatment of chronic inflammatory diseases, including RA, these drugs are effective only in a fraction of patients and have other limitations including adverse effects and a high cost. Given the link between diet and RA, there are a number of studies focusing on certain foods that can help control the inflammation that characterises this autoimmune condition, particularly with respect to cytokine suppression( Reference Rosillo, Alarcón-de-la-Lastra and Sanchez-Hidalgo 9 ). Many of them are found in the so-called Mediterranean diet, which emphasises fish, vegetables and olive oil, among other staples. In fact, the Mediterranean diet has been associated with a reduced incidence of certain pathologies related to chronic inflammation and the immune system( Reference Aparicio-Soto, Sanchez-Hidalgo and Rosillo 10 ). Particularly, extra virgin olive oil (EVOO), the main source of fat in Mediterranean diet, may also help reduce inflammation( Reference Alarcón-de-la-Lastra, Barranco and Motilva 11 ). The health benefits promoted by EVOO cannot only be attributed to its high content in MUFA, but a wide range of evidence indicates that many of the beneficial effects of EVOO intake are owing to its minor highly bioactive components. Among them, phenolic compounds such as hydroxytyrosol (HTy), tyrosol and oleuropein have shown anti-inflammatory and antioxidant effects( Reference Aparicio-Soto, Sanchez-Hidalgo and Rosillo 10 , Reference Cardeno, Sanchez-Hidalgo and Alarcón-de-la-Lastra 12 ).
Our research group has previously demonstrated that treatment with polyphenolic extract (PE) from EVOO or dietary treatment with PE-enriched EVOO diet improved the progression of damage in a collagen-induced arthritis (CIA) model in mice( Reference Rosillo, Alcaraz and Sanchez-Hidalgo 13 , Reference Rosillo, Sanchez-Hidalgo and Sanchez-Fidalgo 14 ). In addition, Impellizzeri et al. ( Reference Impellizzeri, Esposito and Mazzon 15 ) have shown the anti-arthritic effect of oleuropein aglycone in CIA model.
Taking this background into account, the aim of this study was to explore the potential effects of PE from EVOO treatment, in IL-1β-stimulated SW982 cells (human RA-SF cell line), and provide in-depth insight into the signalling pathways involved in its anti-inflammatory effect. Furthermore, the production of proinflammatory mediators, the protein expression of proinflammatory enzymes and the role of MAPK and NF-κB pathways were also determined.
Methods
Reagents
PE from EVOO was obtained by the method described by Vázquez-Roncero et al. ( Reference Vázquez-Roncero, Janer del Valle and Janer del Valle 16 ), with some modifications( Reference Allouche, Jimenez and Gaforio 17 ). For the characterisation, PE (70–75 mg) extracted from EVOO (75 g) was dissolved in CDCl3 (750 µl) or in dimethylsulfoxide (DMSO)-d6 (750 µl) and a precisely measured volume of the solution (550 µl) was placed in a 5-mm NMR tube for the detection and quantification of phenolic compounds. The phenolic mixture to be analysed in DMSO-d6 was previously dissolved in deuterated methanol (MeOD)–D2O 1:1 (1 ml) and concentrated to dryness at reduced pressure in order to exchange hydroxyl protons with 2H nuclei. The NMR solvents were purchased from Sigma-Aldrich. NMR experiments were conducted on a Bruker Avance III 700 spectrometer, operating at 700·25 MHz. The probe temperature was 24·8±1°C. All chemical shifts were given in parts per million (ppm), and the J couplings in Hz. The solvent itself was used as a chemical shift reference (7·26 ppm for CHCl3, and 2·50 for DMSO-d6). 1H NMR spectra were acquired with the following acquisition parameters: acquisition time 2·3 s, relaxation delay 5 s, spectral width of 0–20 ppm, data points 32k, number of scans 32 and line broadening of 0·3 Hz. Post-acquisition processing included zero-filling to 64k. The spectra were phase-corrected automatically using TOPSPIN, and integration was performed manually. We have determined the levels of oleocanthal and oleacein following a modification of the method developed by Karkoula et al. ( Reference Karkoula, Skantzari and Melliou 18 ) for direct measurement of both dialdehydes in olive oil by quantitative high-resolution 1H NMR in CDCl3. The levels of other phenolic compounds have been determined by the procedure described by Christophoridou & Dais( Reference Christophoridou and Dais 19 ). For the experiments, PE from EVOO was dissolved in DMSO and then diluted with the medium (final DMSO concentration ≤0·05 % (v/v)).
Cell culture
The human synovial cell line SW982 was obtained from American Tissue Culture Collection (ATCC®, Number HTB-93). SW982 cells were routinely cultured in T-150 flasks (NuncTM®) and grown in Dulbecco’s modified Eagle’s medium (DMEM) with 2 mm l-glutamine, 10 % fetal bovine serum (FBS) and 1 % penicillin–streptomycin at 37°C, 5 % CO2.
Cell viability assay
Cells seeded in ninety-six-well plates (1×105 cells/well) were incubated in the presence or absence of PE (1·6–200 µg/ml) for 24 h. At the end of the exposure time, the effect of these compounds on cell growth/viability was analysed by sulforhodamine B (SRB) assay( Reference Skehan, Storeng and Scudiero 20 ). After incubation time, adherent cell cultures were fixed in situ by adding 50 µl of 50 % (w/v) cold TCA (Sigma-Aldrich®) and incubated for 60 min at 4°C. The supernatant was discarded, and plates were washed five times with deionised water and dried. In all, 50 µl of SRB (Sigma-Aldrich®) solution (0·4 % w/v) in 1 % acetic acid (Panreac®) was added to each well and incubated for 30 min at room temperature. Plates containing SRB solution were washed five times with 1 % acetic acid. Then, plates were air-dried, and 100 µl/well of 10 mmol/l TRIS base, pH 10·5 (Sigma-Aldrich®), was added, and the absorbance of each well was read on an ELISA reader at 510 nm (BioRad®). Finally, cell survival was measured as the percentage of absorbance compared with that obtained in control cells (non-treated cells).
Determination of matrix metalloproteinase and proinflammatory cytokines by ELISA
SW982 were plated in twenty-four-well plates (2·5×105 cells/well) for 48 h before treatment. Cells were incubated at 37°C in the presence or absence of PE (50, 25 and 12·5 µg/ml) for 24 h in DMEM containing 10 % (v/v) FBS in a 5 % CO2 atmosphere; then, IL-1β (5 ng/ml) was added and incubated for 24 h. Culture supernatants were collected and stored at –80°C. The levels of MMP-1, MMP-3, IL-6 and TNF-α were determined in culture supernatants from the above experiments using commercially available ELISA kits essentially according to the instructions of the manufacturers: MMP-1, RayBiotech®; MMP-3, R&D System®; and IL-6 and TNF-α, eBioscience®.
Immunoblotting detection
Cells (2·5×105 cells/ml) were left untreated or treated with PE (50, 25 and 12·5 µg/ml) for 24 h and stimulated with IL-1β at different time points depending on the protein assayed. After incubation, cells were rinsed, scraped off, collected in ice-cold PBS containing a cocktail of protease and phosphatase inhibitors and processed as described by Sanchez-Hidalgo et al. ( Reference Sanchez-Hidalgo, Martin and Villegas 21 ) to isolate proteins. Protein concentration was measured for each sample using a protein assay reagent (Bio-Rad®) according to Bradford’s method and using γ-globulin as a standard( Reference Bradford 22 ). Aliquots of supernatant containing equal amounts of protein (20 µg) were separated on 10 % acrylamide gel by SDS–PAGE. In the next step, the proteins were electrophoretically transferred into a nitrocellulose membrane and incubated with specific primary antibodies overnight at 4°C: rabbit anti-COX-2 (1:1000) and rabbit polyclonal anti-mPGES-1 (1:200) (Cayman Chemical®); rabbit anti-IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, α) (1:1000), rabbit anti-phosphorylated ERK 1/2 (anti-ppERK1/2) (1:1000) and mouse anti-pERK(1/2) (1:1000) (Cell Signaling®); and mouse anti-pJNK (1:200), rabbit anti-JNK (1:200), mouse anti-p38 mitogen-activated protein kinase (anti-pp38) (1:200) and rabbit anti-p38 (1:200) (Santa Cruz Biotechnology®). After rinsing, the membranes were incubated with a horseradish peroxidase-labelled secondary antibody anti-rabbit (Cayman Chemical®) (1:50 000) or anti-mouse (Dako®) (1:2000) containing blocking solution for 1–2 h at room temperature. To prove equal loading, the blots were analysed for β-actin expression using an anti-β-actin antibody (Sigma-Aldrich®). Immunodetection was performed using enhanced chemiluminescence light-detecting kit (Pierce®). The immunosignals were captured using an LAS-3000 Imaging System from Fujifilm Image Reader (Stamford®), and densitometry data were studied following normalisation to the housekeeping loading control. The signals were analysed and quantified by ImageJ software.
Statistical evaluation
All values in the figures and text are expressed as arithmetic means with their standard errors. Experiments were carried out in triplicate. Data were evaluated with Graph Pad Prism® version 5.01 software. The statistical significance of any difference in each parameter among the groups was evaluated by one-way ANOVA, using Tukey’s multiple-comparisons test as post hoc test. P values <0·05 were considered statistically significant. In the experiments involving densitometry, the figures shown are representative of at least three different experiments performed on different days.
Results
Chemical composition of polyphenolic extract from extra virgin olive oil
Table 1 shows the result of qualitative and quantitative analyses of PE from EVOO. A total of nine different phenolic compounds have been identified, with oleocanthal and oleacein being the main components present in PE.
Table 1 Phenolic content of polyphenolic extract determined by 1H NMR spectroscopy

ppm, Parts per million.
Effects of polyphenolic extract from extra virgin olive oil on cell viability
To evaluate the effects of PE from EVOO on the viability of SW982 cells, the SRB assay was performed. The SRB assay is an efficient method for the toxicity screening of compounds to adherent cells, based on the measurement of cellular protein content. After 24 h, our data demonstrated that the viability of SW982 cells treated with PE was not reduced at concentrations of 1·6–50 µg/ml, showing a cell viability >90 % (Fig. 1).
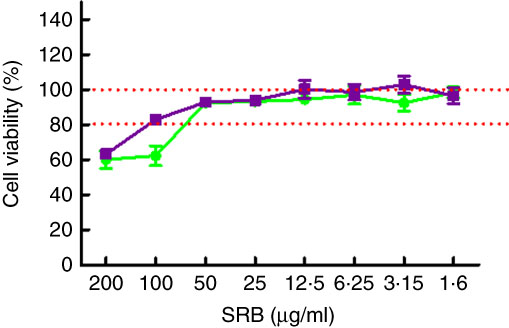
Fig. 1 Effect of polyphenolic extract (PE) from extra virgin olive oil (EVOO) on cell viability. The concentrations used in this study (50, 25 and 12·5 µg/ml) did not affect viability of SW982 cells. Cells were treated with PE from EVOO (1·6–200 µg/ml) for 24 h. Cell survival was measured as the percentage of absorbance compared with that obtained in control cells (non-treated cells). SRB, sulforhodamine B. ![]() , PE;
, PE; ![]() , dimethylsulfoxide.
, dimethylsulfoxide.
Effects of polyphenolic extract from extra virgin olive oil on matrix metalloproteinase production
As shown in Fig. 2, IL-1β at 5 ng/ml significantly increased the MMP-1 and MMP-3 production in SW982 cells. In contrast, pre-treatment with PE for 24 h blocked the IL-1β-induced MMP-1 and MMP-3 up-regulation (Fig. 2).
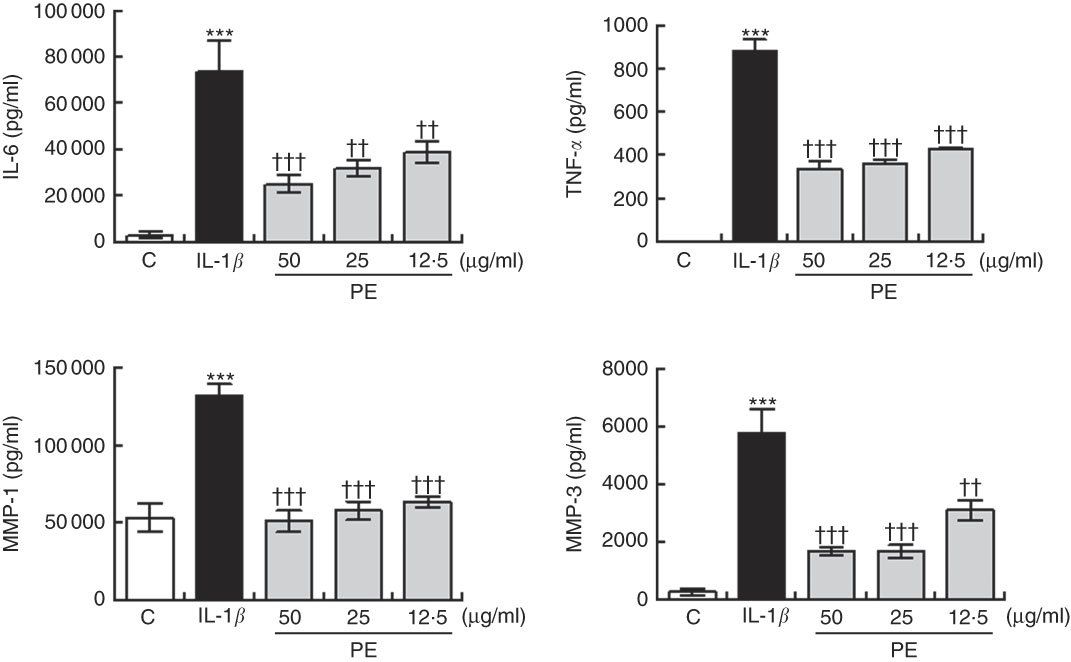
Fig. 2 Inhibitory effects of polyphenolic extract (PE) from extra virgin olive oil on the production of matrix metalloproteinase (MMP) and proinflammatory cytokines by SW982 cells. SW982 cells were cultured for 24 h in the presence or absence of PE and stimulated with IL-1β (5 ng/ml). As controls (C), cells were untreated in the absence of IL-1β. Values are means (n 5), with their standard errors represented by vertical bars. *** Mean values were significantly different from control cells (not stimulated) (P<0·001). Mean values were significantly different from IL-1β-stimulated control cells: †† P<0·01, ††† P<0·001.
Effects of polyphenolic extract from extra virgin olive oil on proinflammatory cytokine production
Treatment of SW982 cells with IL-1β increased the levels of IL-6 and TNF-α when compared with cells in the absence of stimulus (Fig. 2). However, pre-treatment with PE resulted in a significant inhibition of the production of both proinflammatory cytokines induced by IL-1β (Fig. 2).
Polyphenolic extract from extra virgin olive oil down-regulated the cyclo-oxygenase-2 and microsomal PGE synthase-1 over-expression induced by IL-1β
We investigated the possible effects of PE on COX-2 and mPGES-1 inflammation-related enzymes as PG play a crucial role in the pathogenesis of RA. COX-2 and mPGES-1 protein expressions were remarkably increased after 24-h IL-1β stimulation, whereas a significant down-regulation in both pro-inflammatory protein expression was observed in SW982 treated with PE (Fig. 3).
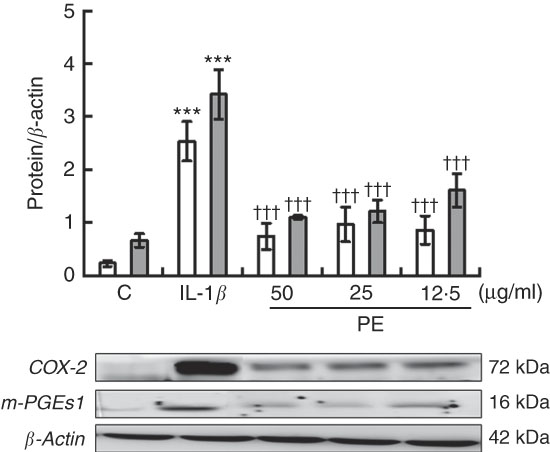
Fig. 3 Polyphenolic extract (PE) from extra virgin olive oil inhibits cyclo-oxygenase-2 (COX-2, ![]() ) and microsomal PGE synthase-1 (m-PGES-1,
) and microsomal PGE synthase-1 (m-PGES-1, ![]() ) protein expressions in IL-1β-stimulated SW982 cells. Cells were untreated or treated with PE (50, 25 or 12·5 µg/ml) for 24 h in the presence of IL-1β (5 ng/ml). Control cells (C) were incubated in the absence of IL-1β. The plots represent band intensity. β-Actin served as an equal loading control for normalisation. Values are means (n 5), with their standard errors represented by vertical bars. *** Mean values were significantly different from control cells (not stimulated) (P<0·001). ††† Mean values were significantly different from IL-1β-stimulated control cells (P<0·001).
) protein expressions in IL-1β-stimulated SW982 cells. Cells were untreated or treated with PE (50, 25 or 12·5 µg/ml) for 24 h in the presence of IL-1β (5 ng/ml). Control cells (C) were incubated in the absence of IL-1β. The plots represent band intensity. β-Actin served as an equal loading control for normalisation. Values are means (n 5), with their standard errors represented by vertical bars. *** Mean values were significantly different from control cells (not stimulated) (P<0·001). ††† Mean values were significantly different from IL-1β-stimulated control cells (P<0·001).
Effects of polyphenolic extract from extra virgin olive oil on IL-1β-induced mitogen-activated protein kinase activation in SW982 cells
To explore possible signalling pathways underlying the anti-inflammatory effects, we evaluated whether PE was able to modulate JNK, p38 and ERK MAPK activation after 30 min of IL-1β stimulation by Western blotting. Our findings demonstrated that SW982 cells stimulated with IL-1β induced remarkably higher levels of phosphorylation of JNK, p38 and ERK MAPK in comparison with unstimulated cells. In contrast, PE treatment was able to reduce the IL-1β-induced phosphorylation of JNK, p38 and ERK MAPK to basal levels similar to those detected in unstimulated cells (Fig. 4).
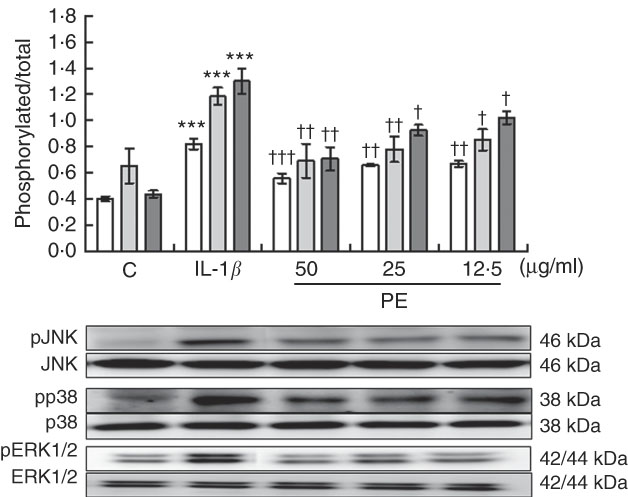
Fig. 4 Effects of polyphenolic extract (PE) from extra virgin olive oil on the mitogen-activated protein kinase signalling pathway in IL-1β-stimulated SW982 cells. Cells were untreated or treated with PE (50, 25 or 12·5 µg/ml) for 24 h and stimulated with IL-1β (5 ng/ml) for 30 min. Control cells (C) were incubated in the absence of IL-1β. Densitometry was performed following normalisation to the control housekeeping genes (JNK, p38 and ERK1/2). Values are means (n 5), with their standard errors represented by vertical bars. *** Mean values were significantly different from control cells (not stimulated) (P<0·001). Mean values were significantly different from IL-1β-stimulated control cells: † P<0·05, †† P<0·01, ††† P<0·001. ![]() , Phospho-c-Jun N-terminal kinase;
, Phospho-c-Jun N-terminal kinase; ![]() , pp38;
, pp38; ![]() , phospho-extracellular signal-regulated kinase 1/2.
, phospho-extracellular signal-regulated kinase 1/2.
Polyphenolic extract from extra virgin olive oil prevented IL-1β-induced IκBα degradation on SW982 cells
NF-κB is a pleiotropic mediator in the control of several inducible and tissue-specific genes and is one of the key regulators of the cellular responses to oxidative stress in mammalian cells. NF-κB activation is initiated by the degradation of IκBα. After IκBα is degraded, NF-κB, in the NF-κB–IκBα complex, is free to be translocated into the nucleus, where it can induce the expression of pro-inflammatory genes contributing to the damage. To determine whether proinflammatory protein down-regulation is regulated by the IκBα pathway, we measured the expression levels of IκBα in SW982 cells pre-treated with PE (24 h) in the presence or absence of stimulus (5 ng/ml IL-1β) for 6 h. As shown in Fig. 5, IκB-α was reduced in SW982 cells stimulated with IL-1β, whereas pre-treatment with PE from EVOO was able to prevent IκB-α degradation in IL-1β-stimulated SW082 cells (Fig. 5).
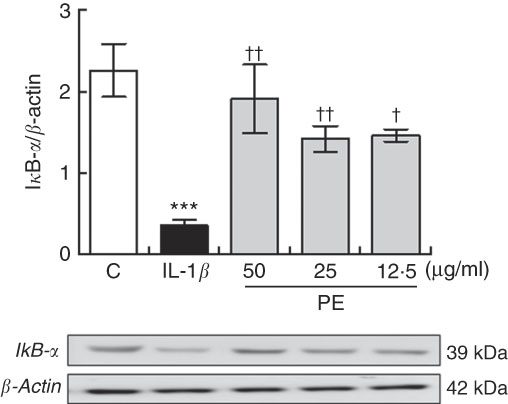
Fig. 5 Polyphenolic extract (PE) from extra virgin olive oil pre-treatment prevented IκB-α degradation in IL-1β-stimulated SW982 cells. Cells were untreated or treated with PE (50, 25 or 12·5 µg/ml) for 24 h and stimulated with IL-1β (5 ng/ml) for 6 h. Control cells were incubated in the absence of IL-1β. Densitometry was performed following normalisation to the control (β-actin housekeeping gene). Values are means (n 5), with their standard errors represented by vertical bars. *** Mean values were significantly different from control cells (no stimulated) (P<0·001). Mean values were significantly different from IL-1β-stimulated control cells: † P<0·05 and †† P<0·01.
Discussion
The development of effective strategies for the prevention and therapy of RA is highly desired. On the basis of our assessment in cultured SW082 SF, PE from EVOO may have promising potential for the development of a novel and effective nutritional supplement for RA suppressing key proinflammatory mediators and cytokines involved in the pathogenesis of RA through blockage of MAPK and NF-κB signalling pathways.
In RA, SF act as a major cell population in the invasive pannus to participate in the chronic inflammatory responses( Reference Firestein 23 ). In the current study, we have shown, for the first time, which PE from EVOO was able to inhibit the activation of SW982 human synovial cells induced by IL-1β, preventing the inflammatory response.
Inflammatory changes of SF play a vital role in the progression of RA. In this sense, it has been reported that synovial reaction in RA patients is characterised by an over-production of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, which are known to play key roles in the pathogenesis of RA( Reference Tanaka 24 ). In addition, it has been demonstrated that an increase of serum c-globulin and the emergence of rheumatoid factors are related to IL-6 levels. In fact, high levels of IL-6 have been detected in both sera and synovial fluids from the affected joints of RA patients( Reference Baillet, Gossec and Paternotte 25 ). On the other hand, TNF-α, a pro-inflammatory agent that is formed in the macrophages and T cells, is reportedly involved in early joint swelling, chronic joint inflammation and the concomitant erosive changes in cartilage and bone( Reference Sommerfelt, Feuerherm and Jones 26 ). In fact, development of TNF-α inhibitors such as infliximab and etanercept has been shown to induce the clinical remission of RA and delay or halt the clinical and radiological progression of the disease, thus improving the quality of life of many patients( Reference Smolen, Landewe and Breedveld 27 ). However, the high cost and the adverse effect profile associated with these drugs limit their wide use as first-line medications. In this context, novel treatment possibilities are necessary for the treatment and/or control of chronic inflammatory state in RA. At present, many dietary phenolic compounds such as curcumin, resveratrol, HTy, oleuropein and epigallocatechin, among others, have reported a beneficial role in the prevention and management of RA, by suppressing the expression of proinflammatory cytokines or mediators, adhesion molecules and MMP in SF( Reference Rosillo, Alarcón-de-la-Lastra and Sanchez-Hidalgo 9 ). In the search of new strategies in RA, the present study confirmed, for the first time, that PE from EVOO treatment caused a significant inhibition of IL-1β-induced TNF-α and IL-6 release in human synovial SW982 cells. These results are in agreement with our previous observations in CIA mouse model, the most commonly studied autoimmune model of RA where PE decreased joint oedema, cell migration, cartilage degradation and bone erosion( Reference Rosillo, Alcaraz and Sanchez-Hidalgo 13 ).
In addition, the cartilage destruction of RA is mostly caused by the activity of MMP, and MMP are pivotal in the recruitment of leucocytes and macrophages into joints. Particularly, MMP-1 (collagenase 1) is one of the principal neutral proteinases that degrade native fibrillar collagens in the extracellular matrix( Reference Yamanishi and Firestein 28 ). Alternatively, MMP-3 (stromelysin) can degrade proteoglycan, type IV and type IX collagens, denatured type I and type II collagens, fibronectin, gelatin and laminin and is considered to be especially important because, in addition to its direct enzyme activity, its activation is necessary for full activation of collagenases( Reference Chen and Mattey 29 ). Our data showed that the production of both MMP-1 and MMP-3 was induced after IL-1β stimulation in human SW982 cells, whereas PE pre-treatment induced a significant down-regulation of both MMP levels in IL-1β-stimulated SW982 cells.
At the same time, cytokine and MMP production directly correlate with increased levels of PG in response to IL-1β in RA-SF( Reference Egg 30 ). Besides, COX-2 and mPGES-1, enzymes responsible for the over-production of PGE2 in inflammation, are up-regulated in RA patients( Reference Lazarus, Kubata and Eguchi 31 ), contributing to the progression of RA through EP4 receptor activation( Reference McCoy, Wicks and Audoly 7 ). In this study, we have shown that PE from EVOO decreased the expression of both COX-2 and mPGES-1 in IL-1β-stimulated SW982 cells. Our data are in agreement with our previous study where we confirmed that PE decreased mPEG-1 and COX-2 protein expression by Western blotting and immunohistochemical staining, respectively, in mice subjected to CIA( Reference Rosillo, Alcaraz and Sanchez-Hidalgo 13 ).
There is evidence that multiple stress signalling pathways, such as MAPK and NF-κB, on activation, act as pivotal regulators of proliferation, differentiation and cellular survival in RA, contributing to the pathogenic mechanisms of joint destruction and inflammation in RA( Reference Liu, Sun and Tao 32 ). It is well known that MAPK and NF-κB pathways are involved in regulating the expression of IL-6, IL-8, MMP-1 and MMP-3 in RA-SF( Reference Han, Boyle and Chang 33 , Reference Tak, Gerlag and Aupperle 34 ) and their signal transduction pathways have been found to be involved in the pathogenesis of RA( Reference Morel and Berenbaum 35 ). Particularly, the three families of MAPK, ERK, JNK and p38 are activated by various mitogens, growth factors and pro-inflammatory cytokines, such as IL-1β and TNF-α. MAPK play a critical role in the regulation of the synthesis of chemokines, cytokines, adhesion molecules and PG involved in RA and are considered as the major tyrosine phosphorylation proteins in human synovial cells stimulated with IL-1β ( Reference Barchowsky, Frleta and Vincenti 36 ). In addition, MAPK modulate MMP production by SF and drive osteoclast differentiation in RA( Reference Han, Boyle and Chang 33 ).
The transcription factor NF-kB has been well recognised as a pivotal regulator of inflammation in RA. It has been evidenced that NF-kB is involved in abnormal apoptosis and proliferation of RA fibroblast-like synovial cells, as well as differentiation and activation of bone resorbing activity of osteoclasts( Reference Makarov 37 ). In RA, NF-κB is over-expressed in the inflamed synovium( Reference Han, Boyle and Manning 38 ), where its activity may enhance recruitment of inflammatory cells and production of proinflammatory mediators such as IL-1β, IL-6, IL-8 and TNF-α ( Reference Tak and Firestein 39 ).
Consistent with other observations, we described that IL-1β enhanced the activation of MAPK; increased the phosphorylation of ERK1/2, JNK and p38; and endorsed IκBα degradation( Reference Castejon, Rosillo and Montoya 40 ). Nevertheless, PE from EVOO pre-treatment prevented both MAPK and NF-κB signalling pathway activation. These results are in concordance with our previous studies in which oral administration of PE from EVOO was able to down-regulate the arthritic process in a CIA murine model blocking these signalling pathways( Reference Rosillo, Alcaraz and Sanchez-Hidalgo 13 ). Similar results were also described by Cardeno et al. ( Reference Cardeno, Sanchez-Hidalgo and Aparicio-Soto 41 ), where PE from EVOO down-regulated efficiently the inflammatory response in lipopolysaccharide-activated murine peritoneal macrophages suppressing NF-κB and MAPK signalling pathways.
Among the components present in the PE from EVOO, oleocanthal was the main component detected in our extract. Nevertheless, although oleocanthal has been shown to exert a key role in the anti-inflammatory and anti-arthritic effects in an in vitro model of degenerative joint disease( Reference Iacono, Gomez and Sperry 42 , Reference Scotece, Gomez and Conde 43 ), other minor bioactive compounds present in the PE, such as HTy and hydroxytyrosol acetate (HTy-Ac), among others, might contribute synergically to these beneficial effects. In fact, previous studies carried out by our research group have demonstrated the anti-inflammatory and joint protective effects of both HTy and HTy-Ac in in vitro ( Reference Rosillo, Sánchez-Hidalgo and Castejón 44 ) and in vivo ( Reference Rosillo, Sanchez-Hidalgo and Gonzalez-Benjumea 45 ) models of RA.
Our study reveals interesting insights into the antioxidant, anti-inflammatory and immunomodulatory properties of PE from EVOO in an establishment in vitro model for human RA, IL-1β-stimulated SW982 cells. These beneficial effects were accompanied by a remarkable reduction of proinflammatory mediators including MMP-1, MMP-3, COX-2, mPGES-1 and cytokines IL-6 and TNF-α. The possible signalling pathways involved in this protective effect could be related to the inhibition of MAPK and NF-κB controlling the production of inflammatory mediators. The observations reported at bench are promising and suggest that the dietary PE from EVOO may influence the course of this disease in humans, ameliorating the RA symptoms and down-regulating the inflammation at the molecular level, opening a new interesting field in which is necessary to explore and bringing them to the forefront of the treatment of this chronic human disease. Nevertheless, it is also important to mention that the bioavailability and the mechanism by which PE from EVOO is absorbed remains unknown and is very limited to their individual compounds( Reference Vissers, Zock and Roodenburg 46 – Reference Tan, Tuck and Stupans 48 ). Therefore, it is necessary to provide deep insight into the processes of absorption and biotransformation of this fraction in order to establish the role of the flora of the colon in these processes and to determine the types of metabolites formed that can contribute to the biological effects finally observed.
Conclusions
PE from EVOO could play an important role in the anti-inflammatory effect of EVOO and probably provide an attractive complement in management of diseases associated with over-activation of SF, such as RA. Nevertheless, future studies should focus more on understanding the biochemical and biological activities of PE from EVOO underlying their effective doses in humans and the dose dependence of their effects.
Acknowledgements
The authors thank I+D+i de Oleoestepa SAC department who kindly provided the EVOO. The authors gratefully acknowledge the assistance of Centre for Technology and Innovation Research, University of Seville (CITIUS).
This work was supported by Junta de Andalucía (P-10AGR-6609). M. A. R. thanks Junta de Andalucía for the award of a pre-doctoral and a post-doctoral grant associated with P-10AGR-6609.
M. A. R. performed the experiments and data analysis. M. L. C, T. M. and M. C.-G. performed part of the analysis. C. A.-d.-l.-L. and M. S.-H. designed the study and prepared the manuscript. All authors read and approved the final content of the manuscript.
The authors declare that there are no conflicts of interest.









