Chronic kidney disease (CKD) is a foremost worldwide public health problem and substantially increases the risk of progression to end-stage renal disease, CVD and mortality(Reference Go, Chertow and Fan1,Reference Peralta, Shlipak and Judd2) . The age-standardised global prevalence of CKD in adults is 11·8 % in women and 10·4 % in men(Reference Mills, Xu, Zhang and Bundy3). It is estimated that over 30 million American adults have CKD, and the prevalence of recognised CKD has steadily risen year after year across all stages of CKD(Reference Saran, Robinson and Abbott4). Therefore, new treatment approaches to this problem are critically needed, and non-traditional risk factors have received considerable interest.
Several observational studies have shown a significant association between high concentrations of plasma total homocysteine and the risk of developing albuminuria and CKD(Reference Ninomiya, Kiyohara and Kubo5–Reference Xie, Yuan and Guo8). However, only one interventional study found that homocysteine-lowering folic acid treatment can significantly delay the progression of CKD among hypertensive patients without folic acid fortification(Reference Xu, Qin, Li and Sun9), and others using high doses of folic acid have shown no benefit or harmful effects on renal outcomes in regions with folic acid fortification(Reference House, Eliasziw and Cattran10,Reference Jamison, Hartigan and Kaufman11) . One interpretation is that chronic exposure to folic acid or high intake of folic acid may induce saturation of the capacity to convert folic acid to the metabolically active 5-methyltetrahydrofolate (5-mTHF), resulting in the presence of unmetabolised folic acid (UMFA) in the circulation(Reference Patanwala, King and Barrett12,Reference Bailey and Ayling13) , which may reduce natural killer cell cytotoxicity(Reference Troen, Mitchell and Sorensen14,Reference Sawaengsri, Wang and Reginaldo15) . Most importantly, previous studies have found that treatment with 5-mTHF v. folic acid improved survival rate in haemodialysis patients(Reference Cianciolo, La Manna and Colì16). Therefore, it is speculated that increased risks associated with UMFA may mask the benefit of 5-mTHF on kidney function. However, to date, few studies have been conducted to assess the association between serum folate forms and the risk of CKD.
To address the above important knowledge gaps, using data from continuous National Health and Nutrition Examination Surveys (NHANES) 2011–2018, we aimed to examine the relation of several serum folate forms (5-mTHF, UMFA and MeFox (pyrazino-s-triazine derivative of 4α-hydroxy-5-methyltetrahydrofolate, an oxidation product of 5-mTHF)) with the risk of CKD and examine any possible effect modifiers in a large and nationally representative sample of USA adults.
Methods
Study design and population
In order to monitor the health and nutritional status of the USA civilian non-institutionalised population, NHANES was conducted every year and released on a 2-year basis by the National Center for Health Statistics of the Centers for Disease Control and Prevention, with a stratified multistage probability sample. Detailed survey operation manuals, consent documents and brochures of each period are available on the NHANES website(17). NHANES was approved by the National Center for Health Statistics Institutional Review Board, and all participants signed an informed consent.
In this study, we analysed data from NHANES 2011–2018 (n 39 156), where a comprehensive list of serum folate forms has been measured by isotope-dilution HPLC coupled to tandem mass spectrometry (LC–MS/MS), and the folate forms concentrations were comparable over time (online Supplementary Table 1)(Reference Fazili, Sternberg and Potischman18).We restricted our analysis to persons who were ≥ 18 years without pregnancy, as well as not receiving dialysis. Of the 23 492 participants, 4735 were excluded due to missing information on serum folate forms concentrations or serum creatinine or urine albumin:creatinine ratio (ACR) at baseline. Therefore, a total of 18 757 subjects were enrolled in our present analysis (online Supplementary Fig. 1).
Measurements of serum folate forms
Specimen donors were recommended fast prior to specimen collection, but fasting is not required. Details on fasting before blood draw were collected via questionnaire before blood draw. Serum samples were analysed for 5-mTHF, UMFA and MeFox using LC–MS/MS by the Centers for Disease Control and Prevention laboratory(Reference Fazili, Whitehead and Paladugula19). Quality control procedures were consistent across cycles, and long-term quality control CV were <3 % for 5-methylTHF and <10 % for UMFA and MeFox(Reference Fazili, Sternberg and Potischman18,20–23) . Imputed values (limit of detection (LOD) divided by square root of two) were used if any folate form result was <LOD. Since UMFA measurements in 2011–2014 were biased about 25 % higher due to issues with folic acid calibrator solubility, the UMFA calibration bias has been corrected mathematically in NHANES 2011–2014 prior to data release and UMFA results produced during NHANES 2015–2018 are based on a modified procedure that avoided the calibration bias(Reference Fazili, Sternberg and Paladugula24,25) .
Ascertainment of kidney function and kidney damage
During NHANES mobile examination centre screenings, serum creatinine was measured using a kinetic rate Jaffe method in NHANES 2011–2016 while using an enzymatic method in NHANES 2017–2018. To appropriately estimate glomerular filtration rate (GFR), regression models were used to correct serum creatinine values in NHANES 2017–2018 as detailed at –https://wwwn.cdc.gov/Nchs/Nhanes/2017–2018/BIOPRO_J.htm. The estimated GFR (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation(Reference Levey, Stevens and Schmid26). Urine albumin concentration was measured in a random, single-voided urine sample using a solid-phase fluorescent immunoassay, and urine creatinine was measured using a Jaffe rate reaction. Urinary ACR was computed and reported in milligrams per gram.
The primary kidney outcome was reduced eGFR, defined as eGFR <60 ml/min/1·73 m2. The secondary outcome was microalbuminuria, defined as an ACR of 30 mg/g to 299 mg/g, and macroalbuminuria, defined as an ACR of 300 mg/g or higher.
Assessments of covariates
Covariates were selected a priori based on biological plausibility and prior empirical evidence associated with folate and kidney function. Detailed information on covariates was available through standardised questionnaires during interviews, including age, sex, race/ethnicity, education level, smoking status, use of folic acid-containing supplements (self-reported use during the 24 h prior to visiting the Mobile Examination Center) and self-reported history of diabetes and hypertension. Baseline body measurement was performed during mobile physical examination. BMI was calculated as weight (kg) divided by the square of height (m2). Serum total cholesterol were measured enzymatically, and HDL-cholesterol was measured by direct immunoassay. Haemoglobin A1c (HbA1c) was measured using high-pressure liquid chromatography.
Statistical analysis
All statistical analyses accounted for complex survey design factors for NHANES, including sample weights, stratification and clustering, following NHANES analytic and reporting guidelines(17). Considering potential non-linear relation of folate forms and kidney outcome and unknown cut-offs for folate forms, we divided folate forms into quartiles for the entire study. Characteristics are presented as mean ± standard error (se) for continuous variables and as proportions for categorical variables. Comparison of characteristics according to quartiles of serum 5-mTHF and UMFA was performed by χ 2 test for categorical variables or ANOVA for continuous variables.
Binomial regression models (i.e. general linear models with the logit link) were used to estimate OR and 95 % CI for kidney outcomes (binary variable, no and yes) according to quartiles of folate forms with the lowest quartile as the reference group. Variables known as traditional or suspected risk factors for kidney disease(Reference Xu, Qin, Li and Sun9), or those showed significant differences among different folate levels were selected as covariables. Therefore, model 1 was adjusted for age (years, continuous) and sex (female and male), and model 2 was further adjusted for BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), education level (less than high school, high school or equivalent and college or above), smoking status (never, past and current), history of diabetes (no and yes) and hypertension (no and yes), total cholesterol (continuous), HDL-cholesterol (continuous) and HbA1c (continuous). We used Bayesian information criterion to determine goodness of fit, and model 2 had lower Bayesian information criterion than crude model and model 1. Furthermore, in order to further improve statistical power, if there were significant relationships between folate forms and outcomes, quartiles were further pooled as post hoc exploratory analyses according to prevalence rate and adjusted OR, to generate binary variables for subsequent subgroup analyses.
To further evaluate whether several folate forms have a confounding effect on one another, stratified analyses by serum 5-mTHF, UMFA and MeFox were performed. As additional exploratory analyses, possible modifications on the relationship of 5-mTHF and UMFA with outcome were assessed for social and demographic variables including age (<55 v. ≥ 55 years), sex, BMI (<25 v. ≥ 25 kg/m2) and current smoker (no v. yes), which were selected a priori based on prior empirical evidence.
A two-tailed P < 0·05 was considered to be statistically significant in all analyses. Analyses were performed using R 3.6.2 software (http://www.R-project.org/) and R package survey.
Results
Baseline characteristics of the participants
Among the 18 757 adult participants, the prevalence of reduced eGFR, microalbuminuria or macroalbuminuria was 6·5 % (n 1438), 8·2 % (n 1875) and 1·4 % (n 398), respectively. Characteristics of the study participants with weighted estimates and unweighted sample sizes according to quartiles of serum 5-mTHF and UMFA are presented in Table 1. The mean values of serum 5-mTHF, UMFA and MeFox were 40·6 nmol/l, 1·9 nmol/l and 1·9 nmol/l, respectively. Participants with folic acid-containing supplements and those without fasting have higher serum folate levels. Among those without folic acid-containing supplements, female have higher 5-mTHF (average 36·3 nmol/l for female and 32·1 nmol/l for male) and UMFA (average 1·13 nmol/l for female and 0·98 nmol/l for male) levels.
Table 1. Baseline characteristics of study participants according to quartiles of 5-methyltetrahydrofolate (5-mTHF) and unmetabolised folic acid (UMFA)*
(Numbers and percentages)
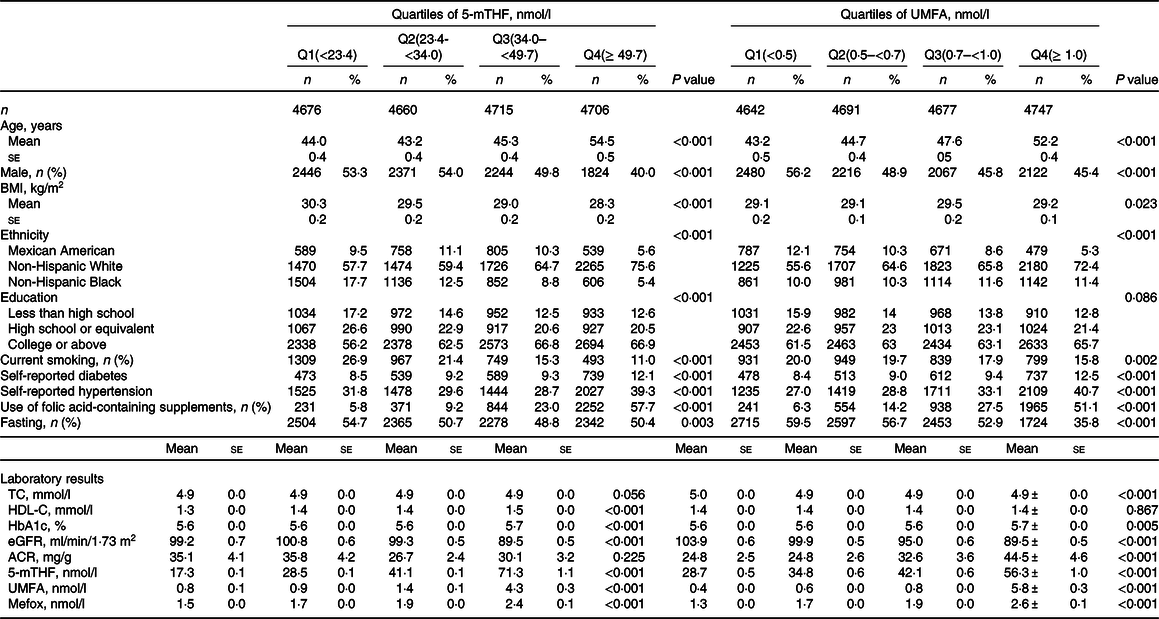
TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; HbA1c, haemoglobin A1c; eGFR, estimated glomerular filtration rate; ACR, albumin:creatinine ratio.
* All estimates accounted for complex survey designs. Values are presented as mean ± se for continuous variables and n (%) for categorical variables, and comparison of characteristics was performed by χ 2 test for categorical variables or ANOVA for continuous variables.
As shown in Table 1, participants with higher level of serum 5-mTHF and UMFA tended to be older, female, non-Hispanic White and more likely to have higher prevalence of self-reported diabetes, as well as have higher level of HbA1c, and MeFox, while less likely to be current smokers. In addition, those with higher level of serum 5-mTHF also trend to have lower BMI, higher HDL-cholesterol, and those with higher level of serum UMFA tended to have higher prevalence of self-reported hypertension, lower level of eGFR and higher level of ACR.
Relationship of folate forms with the risk of Chronic kidney disease outcomes
Overall, there were significant inverse relationships of serum 5-mTHF with reduced eGFR and macroalbuminuria. When serum 5-mTHF was assessed as quartiles, significant lower prevalence of reduced eGFR (OR, 0·71; 95 % CI: 0·57, 0·87) and macroalbuminuria (OR, 0·65; 95 % CI: 0·46, 0·91) were found in participants in quartiles 3–4 (≥ 34·0 nmol/l), compared with those in quartiles 1–2 (<34·0 nmol/l; both P for trend across quartiles <0·05) (Table 2 and Table 3).
Table 2. Relationship of folate forms with reduced estimated glomerular filtration rate (eGFR)*
(Numbers and percentages; odd ratio and 95 % confidence intervals)
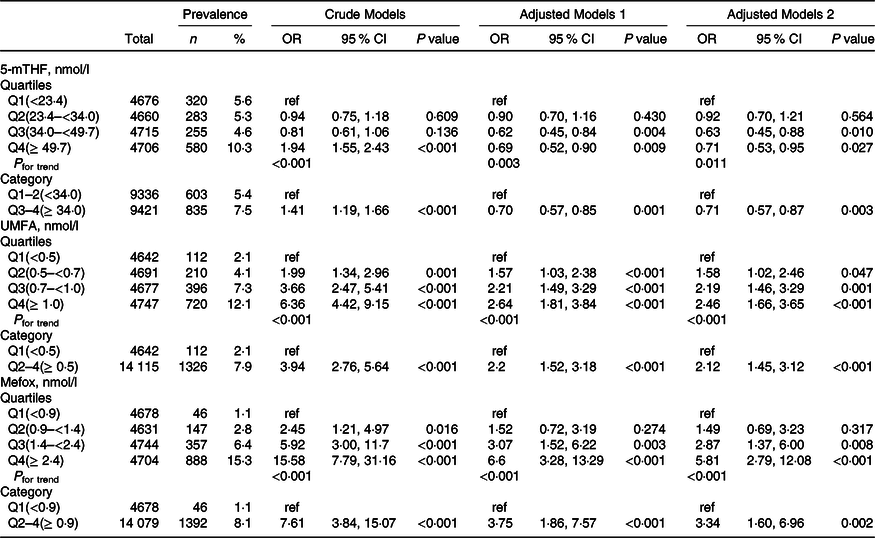
5-mTHF, 5-methyltetrahydrofolate; UMFA, unmetabolised folic acid.
* All estimates accounted for complex survey designs. Binomial regression models were used to estimate OR and 95 % CI, and model 1 was adjusted for age (continuous), sex; model 2 was adjusted for age (continuous), sex, BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), education level (less than high school, high school or equivalent and college or above), smoking status (never, past and current), history of diabetes (no and yes) and hypertension (no and yes), total cholesterol (continuous), high-density lipoprotein cholesterol (continuous) and haemoglobin A1c (continuous). Kidney outcomes were defined as binary variable (no and yes). As primary analysis, folate forms were divided into quartiles, and as exploratory analyses, if the results for primary analysis were significant, quartiles were further pooled to binary variables.
Table 3. Relationship of folate forms with macroalbuminuria*
(Numbers and percentages; odd ratio and 95 % confidence intervals)
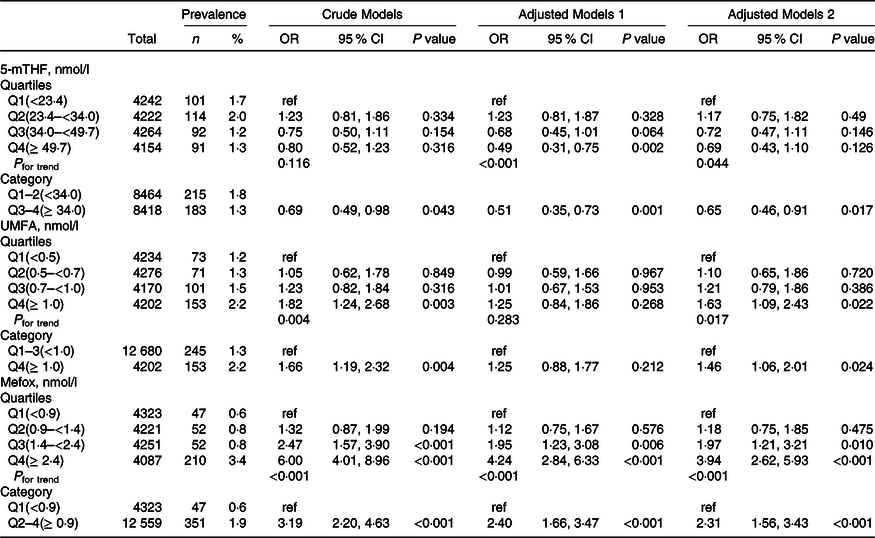
5-mTHF, 5-methyltetrahydrofolate; UMFA, unmetabolised folic acid.
* All estimates accounted for complex survey designs. Binomial regression models were used to estimate OR and 95 % CI, and model 1 was adjusted for age (continuous), sex; model 2 was adjusted for age (continuous), sex, BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), education level (less than high school, high school or equivalent and college or above), smoking status (never, past and current), history of diabetes (no and yes) and hypertension (no and yes), total cholesterol (continuous), high-density lipoprotein cholesterol (continuous) and haemoglobin A1c (continuous). Kidney outcomes were defined as binary variable (no and yes). As primary analysis, folate forms were divided into quartiles, and as exploratory analyses, if the results for primary analysis were significant, quartiles were further pooled to binary variables.
In contrast, there were significant positive relationships of serum UMFA with reduced eGFR and macroalbuminuria. When serum 5-mTHF was assessed as quartiles, significant higher prevalence of reduced eGFR was found in participants in quartiles 2–4 (≥ 0·5 nmol/l; OR, 2·12; 95 % CI: 1·45, 3·12) compared with those in quartile 1 (<0·5 nmol/l; P for trend across quartiles <0·001), and higher prevalence of macroalbuminuria was found in participants in quartile 4 (≥ 1·0 nmol/l; OR, 1·46; 95 % CI: 1·06, 2·01) compared with those in quartiles 1–3 (<1·0 nmol/l; P for trend across quartiles = 0·017) (Table 2 and Table 3). However, there were no significant associations of serum 5-mTHF and UMFA with microalbuminuria (Table 4).
Table 4. Relationship of folate forms with microalbuminuria*
(Numbers and percentages; odd ratio and 95 % confidence intervals)
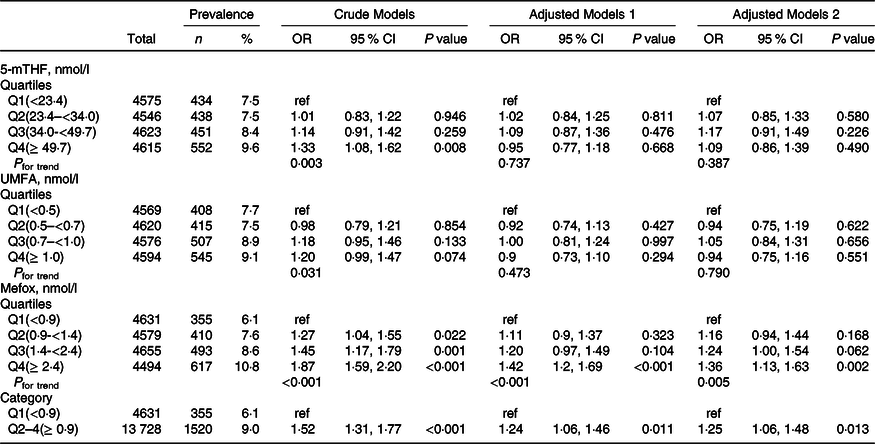
5-mTHF, 5-methyltetrahydrofolate; UMFA, unmetabolised folic acid.
* All estimates accounted for complex survey designs. Binomial regression models were used to estimate OR and 95 % CI, and model 1 was adjusted for age (continuous), sex; model 2 was adjusted for age (continuous), sex, BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and others), education level (less than high school, high school or equivalent and college or above), smoking status (never, past and current), history of diabetes (no and yes) and hypertension (no and yes), total cholesterol (continuous), high-density lipoprotein cholesterol (continuous) and haemoglobin A1c (continuous). Kidney outcomes were defined as binary variable (no and yes). As primary analysis, folate forms were divided into quartiles, and as exploratory analyses, if the results for primary analysis were significant, quartiles were further pooled to binary variables.
More importantly, there were significant positive relationships of serum MeFox with kidney outcomes with significant lower risks of reduced eGFR (OR, 3·34; 95 % CI: 1·60, 6·96), microalbuminuria (OR, 1·25; 95 % CI: 1·06, 1·48) and macroalbuminuria (OR, 2·31; 95 % CI: 1·56, 3·43) in participants in quartile 2–4 (≥ 0·9 nmol/l) compared with those in quartile 1 (<0·9 nmol/l; all P for trend across quartiles <0·01) (Table 2, Table 3 and Table 4).
Stratified analyses
Stratified analyses were performed (Fig. 1 and Fig. 2) to evaluate the relationships of 5-mTHF and UMFA with the prevalence of reduced eGFR and macroalbuminuria in various subgroups. Overall, we did not find any folate forms that significantly modified the association between other folate forms and different kidney outcomes (all P for interactions >0·05).
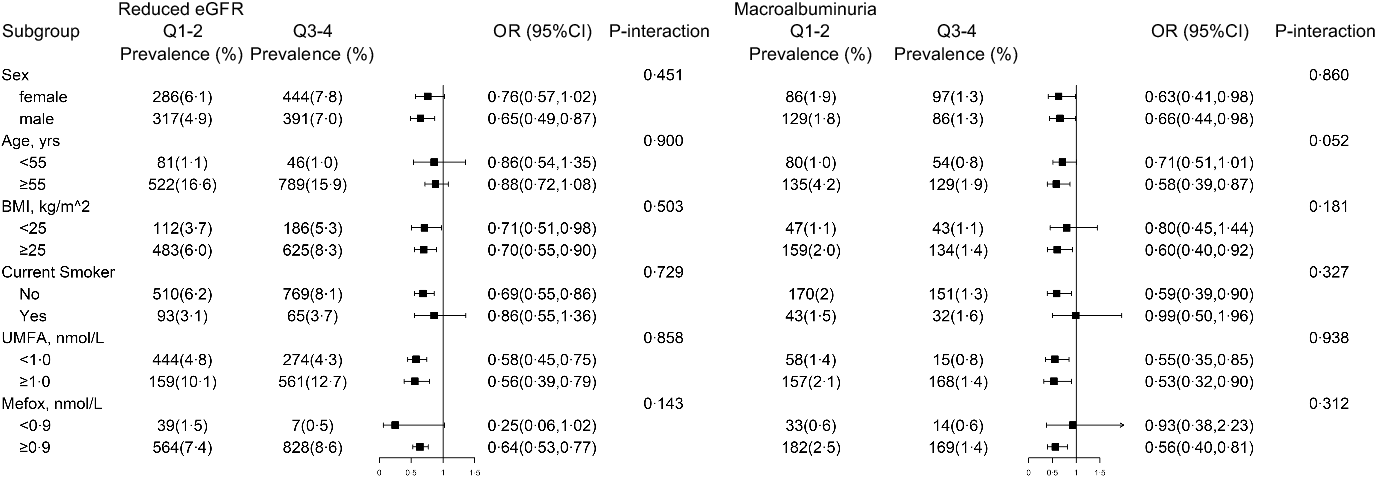
Fig. 1. The association between 5-methyltetrahydrofolate (5-Mthf) and risk of reduced estimated glomerular filtration rate (eGFR) and macroalbuminuria in various subgroups*. * All estimates accounted for complex survey designs. Binomial regression models were used to estimate OR and 95 % CI, and maximum likelihood ratio was used to calculate P value for interaction. Analysis was adjusted for age (continuous), sex, BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), education level (less than high school, high school or equivalent and college or above), smoking status (never, past and current), history of diabetes (no and yes) and hypertension (no and yes), total cholesterol (continuous), HDL-cholesterol (continuous) and haemoglobin A1c (continuous), if not been stratified. Kidney outcomes were binary variable (no and yes), and folate forms were defined as binary variable according to the results of Tables 2–3.
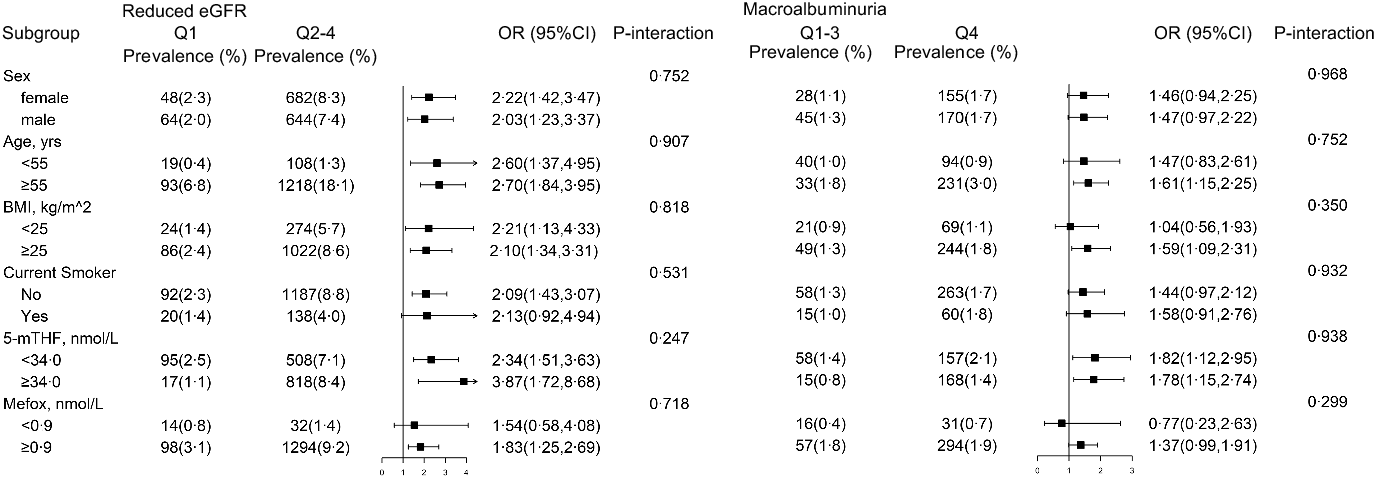
Fig. 2. The association between unmetabolised folic acid (UMFA) and risk of reduced estimated glomerular filtration rate (eGFR) and macroalbuminuria in various subgroups*. *All estimates accounted for complex survey designs. Binomial regression models were used to estimate OR and 95 % CI, and maximum likelihood ratio was used to calculate P value for interaction. Analysis was adjusted for age (continuous), sex, BMI (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and others), education level (less than high school, high school or equivalent and college or above), smoking status (never, past and current), history of diabetes (no and yes) and hypertension (no and yes), total cholesterol (continuous), HDL-cholesterol (continuous) and haemoglobin A1c (continuous), if not been stratified. Kidney outcomes were binary variable (no and yes), and folate forms were defined as binary variable according to the results of Tables 2–3.
Further stratified analyses were conducted to explore other potential interactions (Fig. 1 and Fig. 2), and none of the variables, including age, sex, BMI and smoking status, significantly modified the association between folate forms and different kidney outcomes (all P for interactions >0·05).
Discussion
In a large and nationally representative, randomly selected sample of USA adults, we first demonstrated that high serum 5-mTHF levels were associated with decreased risk of reduced eGFR and macroalbuminuria, while high serum UMFA and MeFox were associated with increased risk of reduced eGFR and macroalbuminuria. Most importantly, none of any folate forms significantly modified the association between other folate forms and renal outcome.
Indeed, synthetic folic acid might have different biological effects than naturally occurring folates. First, there are differences in the bioavailability of 5-mTHF and synthetic folic acid. Folic acid lacks any coenzyme activity until it is reduced to the metabolically active tetrahydrofolate form, and the reduction process was catalysed by dihydrofolate reductase(Reference Pietrzik, Bailey and Shane27), whose activity varied considerably between individuals and was inhibited by UMFA(Reference Bailey and Ayling13). In addition, high UMFA level also inhibited methylenetetrahydrofolate reductase (MTHFR), the enzyme that produces methyl tetrahydrofolate(Reference Christensen, Mikael and Leung28), and thus decrease the bioavailability of 5-mTHF levels. In contrast, the bioavailability of 5-MTHF was higher than that of UMFA, irrespective of the patient’s genotype(Reference Willems, Boers and Blom29). Second, previous studies have reported that UMFA was associated with neurological and cognitive effects in individuals with lower B12 status(Reference Morris, Jacques and Rosenberg30) and with reduced natural killer cell cytotoxicity in some studies in vivo (Reference Troen, Mitchell and Sorensen14,Reference Sawaengsri, Wang and Reginaldo15) . In addition, UMFA has a poor antioxidant activity in peroxynitrite scavenging and lipid peroxidation inhibition(Reference Rezk, Haenen and van der Vijgh31). On the contrary, 5-mTHF had the most prominent antioxidant activity, especially in peroxynitrite scavenging and superoxide production inhibition(Reference Rezk, Haenen and van der Vijgh31,Reference Hyndman, Verma and Rosenfeld32) . Additionally, the structure of 5-mTHF was similar to tetrahydrobiopterin (BH4), which was necessary to maintain endothelial nitric oxide synthase with a net production balance towards nitric oxide, and thus 5-mTHF can restore nitric oxide-dependent endothelial function(Reference Hyndman, Verma and Rosenfeld32,Reference Stroes, van Faassen and Yo33) . As such, it is necessary to examine the potential different health effects of circulating 5-mTHF and UMFA.
Our previous study has observed a U-shaped association of serum 5-mTHF with mortality, while a positive association of UMFA with mortality(Reference Liu, Zhang and Zhou34). However, the association of 5-mTHF and UMFA with renal outcomes remains unclear. To our knowledge, this is the first study to examine the relation of serum 5-mTHF and UMFA levels with renal outcomes, and we found an opposite effect of serum 5-mTHF and UMFA on reduced eGFR and macroalbuminuria. In addition, although there were differences in concentrations of folate forms by fasting status or use of folic acid-containing supplements(Reference Fazili, Sternberg and Potischman18,Reference Pfeiffer, Sternberg and Fazili35) , fasting status (yes v. no) or use of folic acid-containing supplements (yes v. no) did not materially modify the relationship of 5-mTHR or UMFA with study outcomes (online Supplementary Table 2 and Table 3). Of note, in NHANES 2011–2014 (vitamin B12 was not available for 2015–2018), the prevalence of metabolic vitamin B12 deficiency (<248 pmol/l) was 16·4 %. Since vitamin B12 deficiency may lead to methyl folate trap, we categorised data from NHANES 2011–2014 by vitamin B12 deficiency and found that the relationships of 5-mTHF with reduced eGFR and macroalbuminuria were more obvious in those without vitamin B12 deficiency, although the P value for interaction was not significant (online Supplementary Table 2 and Table 3). Along with our previous study(Reference Li, Spence and Wang36), these findings suggest the importance of maintaining relatively higher levels of both 5-mTHF and vitamin B12 on kidney outcomes.
To date, few interventional studies have explored the effect of folic acid supplementation on CKD progression, and the results remain inconsistent. While the CSPPT Renal Substudy demonstrated statistically significant reductions in the risk of CKD progression with low-dose folic acid supplementation (0·8 mg/d) in a hypertensive population without dietary fortification of folic acid(Reference Xu, Qin, Li and Sun9), the HOST study reported that treatment with high doses of folic acid (40 mg/d) did not delay the time to initiating dialysis in patients with advanced CKD or end-stage renal disease(Reference Jamison, Hartigan and Kaufman11), and, even, the DIVINe trial showed that folic acid (2·5 mg/d) treatment resulted in a greater decrease in eGFR in 238 patients with diabetic nephropathy(Reference House, Eliasziw and Cattran10). A possible explanation was that the latter two trials were conducted in regions with folic acid fortification and used high dose of folic acid, as such, much of the folate in the blood would be UMFA(Reference Pfeiffer, Sternberg and Fazili37), which was associated with increased risk of reduced eGFR and macroalbuminuria as shown in our study. Moreover, it should be noted that CKD individuals may be asked to restrict P or K(Reference Ikizler, Burrowes and Byham-Gray38), as well as whole grains or certain fruits and vegetables because of their high P or K content(Reference Williams, Ronco and Kotanko39,Reference Cases, Cigarrán-Guldrís and Mas40) . Since food folates occur naturally in richest supply in green leafy vegetables, while folic acid is found in the human diet in folic acid fortified grain products(Reference McNulty, Ward and Hoey41), P or K-restricted diet offered to patients with CKD may be associated with declining folate intake. As such, our study may possibly overestimate the benefit of 5mTHF and underestimate the hazard of UMFA. Therefore, our result speculated that UMFA may mask the benefit of 5-mTHF on renal outcomes and suggested that 5-mTHF should replace folic acid in future studies of B vitamin supplementation on CKD.
A notable finding from this study is the null association of 5-mTHF and UMFA with microalbuminuria, whereas a significant opposite association of 5-mTHF and UMFA with macroalbuminuria. Our previous study has found that the presence of CKD at baseline was a significant modifier of the treatment effect of folic acid supplement, with a significant renal protective effect in those with mild-to-moderate CKD, whereas a nominal effect in those without CKD(Reference Xu, Qin, Li and Sun9). Since high-grade proteinuria is an independent mediator of progressive kidney damage(Reference Gorriz and Martinez-Castelao42), it was speculated that 5-mTHF and UMFA play a role on promoting the progression of already-initiated CKD rather than against CKD initiation. Another interpretation was potential misclassification of microalbuminuria. A subsample of 1241 NHANES III participants was reexamined 2 weeks after the initial examination, and all sampled participants with macroalbuminuria at the first visit had have persistent albuminuria; however, only 63·2 % participants with microalbuminuria at the first visit had micro- or macroalbuminuria at the second visit(Reference Coresh, Selvin and Stevens43,Reference Coresh, Astor and Greene44) . Therefore, future studies with repeated measurements of albuminuria are needed to verify our results.
Our study additionally sought to evaluate the association of serum MeFox, an oxidation product of 5-mTHF that lacks vitamin biologic activity(Reference Gregory45), with renal outcomes. While it has been shown that MeFox can be formed in vitro after blood collection(Reference Hannisdal, Ueland and Svardal46), some observations raised the possibility that MeFox may be formed in vivo rather than solely in vitro as part of 5-mTHF oxidation(Reference Fazili, Sternberg and Potischman18,Reference Pfeiffer, Sternberg and Fazili35,Reference Paniz, Lucena and Bertinato47) . Therefore, it was suggested to separately report results for 5-methylTHF and MeFox to provide broader utility, given that MeFox data may provide relevant information regarding the quality of sample handling and insights into folate metabolism. Indeed, more MeFox generation or accumulation in older persons, obese adults and smokers, and MeFox concentrations were positively associated with inflammation, suggesting that MeFox may be an indicator of ageing and negative health factors(Reference Fazili, Sternberg and Potischman18,Reference Pfeiffer, Sternberg and Fazili35) . Consistently, our study found a positive relationship of MeFox with reduced eGFR and albuminuria, and underlying mechanisms is required to further examine.
This study had several strengths, including its large and nationally representative sample, the central lab and standardised measurement methods, the relatively higher population’s folate status, the availability of data on several serum folate forms and the comprehensively adjustments for potential confounders. However, our study also has several limitations. First, the cross-sectional design precludes the ability to assess causality. However, although impairment in renal reabsorption of folates in the proximal tubules can lead to a state of folate deficiency(Reference Samodelov, Gai and Kullak-Ublick48), it is highly unlikely that reduced eGFR or albumin loss in urine causes lower 5-mTHF, but higher UMFA. Moreover, our previous study did observe that folic acid therapy can significantly delay the progression of CKD among patients with mild-to-moderate CKD(Reference Xu, Qin, Li and Sun9). At the same time, our current study further found that albuminuria (ACR < 30 v. ≥ 30 mg/g) did not significantly affect the relationship of 5-mTHF with the prevalence of reduced eGFR or macroalbuminuria (online Supplementary Table 2). As such, our findings may possibly indicate that higher 5-MTHF is associated with less risk of kidney damage. However, future cohort studies are necessary to confirm our findings. Second, the folate forms were based on one-time assessment, which may not accurately represent long-term folate levels; however, since folate forms in red blood cell were not available, further studies regarding intracellular folate forms are needed. Third, our study was conducted in USA adults with folic acid fortification. Whether the findings can be extrapolated to other populations will require further investigation. Fourth, we did not have detailed information about MTHFR genotypes, so that we could not assess the possible effect of MTHFR genotypes on the association between folate and kidney outcomes. Fifth, since our study was an exploratory analysis, we did not account for multiple testing. Overall, a more comprehensive picture provided by further studies is required.
Conclusions
In conclusion, using data from a large, nationally representative cohort of USA adults, we found that higher level of 5-mTHF was associated with lower risk of reduced eGFR and macroalbuminuria, whereas higher level of UMFA and MeFox were associated with higher risk of reduced eGFR and macroalbuminuria. Given that folic acid fortification is common in many countries, and there is a high rate of supplement use (approximately 36·6 % among USA adults)(Reference Chen, Du and Blumberg49), our findings, if further confirmed by future studies, emphasised the importance of monitoring the folate forms concentrations and may help counsel future related clinical trials and nutritional guidelines regarding the folate supplement on CKD.
Acknowledgements
The authors thank the investigators and participants of the National Health and Nutrition Examination Survey, the parent study, who made this report possible.
The study was supported by the National Natural Science Foundation of China (81973133, 81730019) and Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009).
M. L. and X. Q. designed the research; M. L. and C. Z. analysed the data; M. L. and X. Q. wrote the paper. All authors contributed to data collection and reviewed/edited the manuscript for important intellectual content. All authors read and approved the final manuscript.
No disclosures were reported.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521001665











