Stearic acid (SA; C18:0) is a long-chain fatty acid (FA) currently available as a fat supplement fed to increase dietary energy density to support the energy needs for lactation(Reference Piantoni, Lock and Allen1). Although its supplementation would be expected to increase preformed FA supply to the mammary gland and milk fat synthesis, research using C18:0 in dairy cows has shown contrasting results. Some studies have reported increased milk yield and/or fat content and yield in dairy cows(Reference Piantoni, Lock and Allen1–Reference Steele3).
Additionally, C18:0 supplementation has been studied as a way to recover from milk fat depression (MFD) induced by fish oil in lactating sheep, albeit without success(Reference Toral, Hervás and Carreño4,Reference Toral, Hervás and Frutos5) . MFD is characterised by a specific reduction in milk fat synthesis without any effect on other milk components(Reference Bauman and Griinari6), being associated with the production of intermediate FA from ruminal biohydrogenation, such as the trans-10, cis-12 isomer of the conjugated linoleic acid (CLA)(Reference Bauman, Perfield and Havartine7).
The effects of C18:0 on the recovery from MFD under other models of induction, such as trans-10, cis-12 CLA, have not been specifically investigated. Indeed, information is lacking on the effect of C18:0 supplementation on the fat content, milk FA profile and potential to recover from trans-10, cis-12 CLA-induced MFD, as well as potential effects on lipogenic gene expression in the mammary gland of dairy ewes. Inhibition of milk fat synthesis by the administration of trans-10, cis-12 CLA is mediated, at least in part, by down-regulating the gene expression of lipogenic enzymes and transcription factors in the mammary gland. Such effects have been demonstrated in several species of domestic animals, such as cows(Reference Baumgard, Matitashvili and Corl8), sheep(Reference Ticiani, Urio and Ferreira9), goats(Reference Zhang, Huang and Tian10) and sows(Reference Sandri, Harvatine and Oliveira11).
The objectives of this study were to evaluate the effects of C18:0 supplementation on milk fat synthesis and the mammary expression of lipogenic genes during CLA-induced MFD. Our hypothesis was that feeding SA to lactating ewes would increase the fat content in milk in a normal scenario and supply preformed FA to the mammary gland, helping in recovering milk fat synthesis, countering the antilipogenic effects of trans-10, cis-12 CLA in a CLA-induced MFD.
Material and methods
Animals and treatments
The Ethics Committee on Animal Experimentation at the Santa Catarina State University approved all procedures by protocol 5642240818. Twenty-eight multiparous Lacaune ewes, averaging 36 (sd 2) days in lactation, 1·8 (sd 0·4) kg of milk/d, 70·5 (sd 9·6) kg of body weight and 3·0 (sd 0·5) of body condition score (BCS) were distributed in four homogeneous groups in a completely randomised design. The experimental period lasted 21 d, with 7 d of adaptation and 14 d of data and sample collection. The number of experimental units per treatment was based on similar study(Reference Ticiani, Urio and Ferreira9) in order to allow detection of significant differences for main parameters in the present study.
All animals were housed in stalls with individual feeders and received one of the following treatments: (1) Control (no lipid supplementation, 25 ml of oral water); (2) CLA (6·4 g/d of trans-10, cis-12 CLA, supplied orally); (3) SA (28 g/d of C18:0, supplied individually in the concentrate and 25 mL of oral water) and (4) SA in association with trans-10, cis-12 CLA (CLASA; 6·4 g/d of trans-10, cis-12 CLA plus 28 g/d of C18:0). The lipid supplements (online Supplementary Table S1) used were Prius F100 Nat Dry (87 % C18:0; Auster Animal Nutrition) and (Luta-CLA 60; BASF AG, containing 29·9 % of trans-10, cis-12 and 29·8 % of cis-9, trans-11 CLA).
The diet consisted of maize silage and concentrate (58 % ground maize, 38 % soybean meal and 4 % mineral-vitamin premix), and the chemical composition is reported in online Supplementary Table S2. Each animal received 1·8 kg of concentrate/d (as-fed basis) in two meals after milking. The SA supplement (14 g) was mixed with 200 g of concentrate to ensure complete intake of the FA, and the remaining was supplied to provide the whole meal (900 g). In order to not restrict voluntary intake, maize silage was supplied at 32 kg/group per d (as-fed basis) in two meals, immediately after the concentrate feeding. The trans-10, cis-12 CLA was supplied orally before the morning milking. The animals in the Control and SA treatments were orally dosed with 25 ml of water, ensuring the same nutritional management for all animals.
Diet was calculated according to the Small Ruminant Nutrition System(Reference Tedeschi, Cannas and Fox12). The doses of trans-10, cis-12 CLA and SA were according to Oliveira et al. (Reference Oliveira, Gama and Fernandes13) and Toral et al. (Reference Toral, Hervás and Carreño4), respectively. Feed refusals were weighed after each meal to determine dry matter intake. Animal weight and BCS assessments were performed individually on days 0 and 20 of the experimental period, and the BCS was measured by one trained observer as described by Russel et al. (Reference Russel, Doney and Gunn14). One ewe from the CLA group was removed in the middle of the experiment due to lameness.
Milk production, composition and fatty acid profile
Milk yield was recorded individually twice a day at 06.00 and 14.00, and milk samples were collected on day 0 of the experimental period and after every 2 d, stored at 4°C with preservative (Bronopol; D & F Control Systems Inc.) before being analysed for composition (fat, protein, lactose and total solids) by IR spectrometry (DairySpec; Bentley Instruments Inc.). Additional samples were collected on day 20 and frozen at −20°C without preservative for FA analysis. All milk samples were composites from morning and afternoon milking.
Milk fat was extracted by centrifugation at 3000 rpm, for 15 min at 4°C, and then methylated according to O’Fallon et al. (Reference O’Fallon, Busboom and Nelson15). The FA methyl esters were determined by GC (Focus GC; Thermo Scientific), following Kramer et al. (Reference Kramer, Fellner and Dugan16), and the FA were identified using three reference standards (Supelco FAME mix # C4-C24, trans-9, cis-11 CLA # 16413, and trans-10, cis-12 CLA # 04397; Sigma Aldrich Inc). In addition, FA desaturase indexes were calculated according to Kelsey et al. (Reference Kelsey, Corl and Collier17).
Biopsy, RNA extraction, complementary DNA synthesis (cDNA) and real-time quantitative PCR
Biopsies on the mammary gland were performed on the last day of the experimental period in twenty-four animals (six sheep/treatment). Previously, udder asepsis was performed with an iodised alcohol solution (10 %) and local anaesthesia (8 ml of lidocaine hydrochloride). Approximately 50 mg (sd 0·01) of tissue/animal was collected, using a coaxial needle (Hospifer) and a biopsy tool (Geotek Medikal, Estacore disposable biopsy cannula). Samples were immediately washed with phosphate-buffered saline solution and stored in liquid N2. After the procedure, the ewes received anti-inflammatory (2 ml of flunixin meglumine), antibiotic (2 ml of ceftiofur hydrochloride) and were manually milked for 5 d to remove possible blood clots.
The total RNA was extracted using the RNeasy Lipid Tissue Kit (Qiagen Sciences), with ‘on column’ DNase treatment to remove possible DNA residues (Sigma-Aldrich). The concentration of RNA was verified using a spectrophotometer (NanoDrop, ND-2000; Thermo Fisher Scientific) and purity by the A260/280 ratio (2·05 (sd 0·02)). Total RNA was transcribed to complementary DNA (cDNA) using GoScript™ Reverse Transcription Mix and random primers (Promega Corporation).
The real-time quantitative PCR analysis was performed according to the protocol described by Ticiani et al. (Reference Ticiani, Urio and Ferreira9). The primer sequences used (online Supplementary Table S3) were obtained from studies previously published or designed in GenBank (NCBI) and synthesised by Invitrogen. They were also validated for specificity, linearity and efficiency for each gene of interest. The evaluated genes were: acetyl-CoA carboxylase α promoter II (ACACAα PII), fatty acid synthase, FA translocator (CD36), lipoprotein lipase, stearoyl-CoA desaturase 1, fatty acid-binding proteins 3 and 4, glycerol-3-phosphate acyltransferase, acylglycerolphosphate acyltransferase 6, diglyceride acyltransferase 1, sterol regulatory element-binding protein 1, PPAR-γ and the housekeeping genes actin-β and ribosomal protein S18.
Statistical analysis
All data were analysed using the MIXED procedure of SAS University Edition (SAS Institute, Inc.). Treatment was considered as a fixed effect, and the animal as a random in the statistical model. Data on daily dry matter intake, milk production and composition were analysed using repeated measures, where the animal was the subject of the repeated statement. Milk production and composition measured on day ‘zero’ of the experimental period were used as covariate. The gene expression analysis was run using the data for each gene in each animal divided by the geometric mean of two housekeeping genes (ribosomal protein S18 and actin-β). When necessary, data were log transformed, and back transformed data were reported. The studentised residuals outside the range of ± 2·5 units were considered outliers and excluded from the analysis. Least-Square Means was used to compare treatments and significance was declared at the level of 5 %, and trend between 5 and 10 %.
Results
Dry matter intake and animal performance
The CLA treatment decreased concentrate intake by 10·4 % (P = 0·001) compared with the average of the other treatments (Table 1). The SA reduced (P = 0·0001) silage intake, followed by the CLA treatment, with no differences for Control and CLASA (Table 1). No changes were observed on BCS and body weight among treatments.
Table 1. Effect of stearic acid supplementation and MFD induction by trans-10, cis-12 CLA of dairy ewes on lactation performance

MFD, milk fat depression; CLA, conjugated linoleic acid; DMI, dry matter intake; SA, stearic acid; CLASA, stearic acid in association with trans-10, cis-12 conjugated linoleic acid.
Averages followed by different lowercase letters differ from each other (P < 0·05).
* Treatments were Control (CONT, n 7): basal diet, CLA (6.4 g/animal per d trans-10, cis-12 CLA, n 7), SA (28 g/animal per d C18:0, n 7), CLASA (6.4 g of trans-10, cis-12 CLA and 28 g of C18:0 animal/d, n 7).
† Standard error of the mean.
‡ Probability for the fixed effects of treatments.
§ Dry matter intake of concentrate was calculated individually.
|| Dry matter intake of silage was calculated by treatment.
¶ Estimated dry matter intake per animal/treatment, as follows: dry matter intake by treatment/number of animals per treatment = kg/animal per d.
** Scale 1–5 as described in the study by Russel et al. (Reference Russel, Doney and Gunn14).
Milk yield and composition
No treatment effect was observed for the yield of milk, protein, lactose and total solids, and for protein and lactose concentration (Table 1). Relative to the Control, the SA treatment had no effect on milk fat yield and content (P = 0·87) or on the total solid content (P = 0·99). Milk fat content and yield were reduced (P = 0·0001) by both CLA and CLASA treatments. The CLASA had the lowest milk total solid content, followed by the CLA treatment, with no differences between Control and SA (Table 1).
Milk fatty acid secretion
Compared with SA, the CLASA treatment reduced the secretion of saturated FA with 6, 8, 10 and 12 carbons by 0·5 (P = 0·03), 1·2 (P = 0·01), 43 (P = 0·01) and 67 % (P = 0·03), respectively (Table 2). The content of palmitoleic acid was reduced by 36 % for CLASA (P = 0·01) compared with SA.
Table 2. Effect of stearic acid supplementation and MFD induction by trans-10, cis-12 CLA of dairy ewes on the milk fatty acid secretion
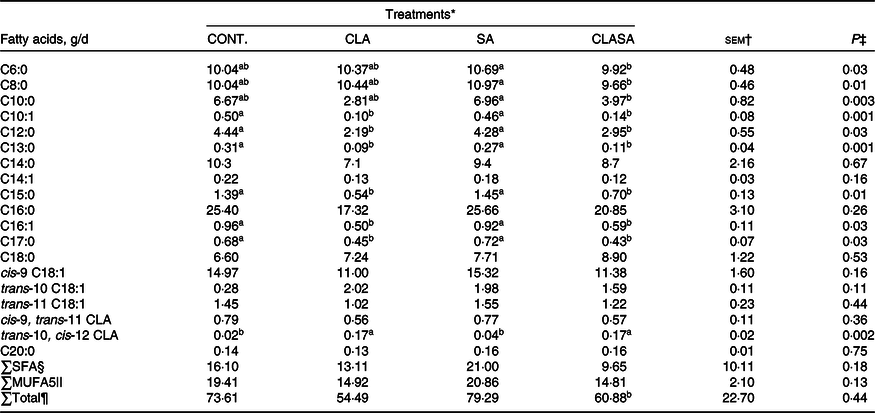
MFD, milk fat depression; CLA, conjugated linoleic acid; SA, stearic acid; CLASA, stearic acid in association with trans-10, cis-12 conjugated linoleic acid.
Averages followed by different lowercase letters differ from each other (P < 0·05).
* Treatments were Control (n 7) in the basal diet, CLA (6.4 g/animal per d trans-10, cis-12 CLA; n 7), SA (28 g/animal per d C18:0; n 7) and CLASA (6.4 g of trans-10, cis-12 CLA and 28 g of C18:0 animal/d; n 7).
† Standard error of the mean.
‡ Probability for the fixed effects of treatments.
§ SFA secretion.
|| MUFA secretion.
¶ Total fatty acids secretion.
The secretions of saturated FA and C18:0 did not differ among treatments. The secretion of trans-10, cis-12 CLA was increased in the CLA and CLASA treatments by 325 % (P = 0·005) compared with the SA treatment. In addition, the secretion of C18:1 trans-10 was not different among treatments (P = 0·11) (Table 2).
In addition, the SA, CLA and CLASA treatments reduced the C16:0/C16:1 and C18:0/C18:1 desaturase indexes by 33 (P = 0·03) and 9 % (P = 0·01), respectively (online Supplementary Table S4).
Gene expression of the mammary gland
The ACACAα PII mRNA abundance was reduced by 45 % (P = 0·0001) in the SA when compared with Control. The ACACAα PII was also reduced with the CLA and SA treatments compared with CLASA by 39 (P = 0·002) and 24 % (P = 0·0001), respectively (Fig. 1(a)). The abundance of fatty acid synthase mRNA was reduced by 27 % (P = 0·08) for the CLASA treatment compared with SA (Fig. 1(b)).

Fig. 1. Relative abundance of ACACAα PII (a) and FASN (b) mRNA, involved in de novo synthesis in the mammary gland of lactating ewes. Values are presented as means with bars representing the sem. Lower case letters differ from each other (P < 0·05). Treatments were Control, CLA (6·4 g/animal per d trans-10, cis-12 CLA), SA (28 g/animal per d C18:0) and CLASA (6·4 g of trans-10, cis-12 CLA and 28 g of C18:0 /animal per d). ACACAα PII, acetyl-CoA carboxylase; FASN, fatty acid synthase.
The lipoprotein lipase mRNA abundance was reduced by 26 % (P = 0·05) in the CLASA treatment compared with Control (Fig. 2(a)). The SA increased the abundance of CD36 mRNA by 78 (P = 0·01) and 140 % (P = 0·01) compared with Control and CLASA, respectively (Fig. 2B).

Fig. 2. Relative abundance of LPL (a), CD36 (b), FABP4, (c) and SCD1 (d) mRNA, involved in the capture, transport and desaturation of fatty acids in the mammary gland of lactating ewes. Values are presented as means with bars representing the sem. Lower case letters differ from each other (P < 0·05). Treatments were Control, CLA (6·4 g/animal per d trans-10, cis-12 CLA), SA (28 g/animal per d C18:0) and CLASA (6·4 g of trans-10, cis-12 CLA and 28 g of C18:0/animal per d). LPL, lipoprotein lipase; CD36, fatty acid translocator; FABP4, fatty acid binding protein 4; SCD1, stearoyl-co-enzyme A desaturase 1.
The abundance of fatty acid-binding protein 4 mRNA was increased by 112 % (P = 0·01) in the SA treatment compared with CLASA (Fig. 2(c)). Compared with Control, stearoyl-CoA desaturase 1 mRNA abundance was reduced by 40 (P = 0·003), 38 (P = 0·005) and 37 % (P = 0·003) for SA, CLA and CLASA, respectively (Fig. 2(d)).
The abundance of PPAR-γ mRNA observed for the SA treatment increased by 39 % (P = 0·03) when compared with Control (Fig. 3(a)). The CLASA treatment reduced the abundance of PPAR-γ mRNA compared with SA by 40 % (P = 0·003; Fig. 3(a)). Relative to Control, CLA and CLASA treatments reduced the abundance of sterol regulatory element-binding protein 1 mRNA by 40 (P = 0·006) and 25 % (P = 0·06; Fig. 3(b)), respectively.

Fig. 3. Relative abundance of the PPAR-γ (a) and SREBP1 (b) mRNA, involved in gene regulation in the mammary gland of lactating ewes. Values are presented as means with bars representing the sem. Lower case letters differ from each other (P < 0·05). Treatments were Control, CLA (6·4 g/animal per d trans-10, cis-12 CLA), SA (28 g/animal per d C18:0) and CLASA (6·4 g of trans-10, cis-12 CLA and 28 g of C18:0 /animal per d). SREBP1, sterol regulatory element-binding protein 1.
Discussion
We evaluated the potential of C18:0 supplementation to increase milk fat synthesis with traditional diets and under milk fat-depressing conditions induced by trans-10, cis-12 CLA. Importantly, our MDF induction model using a CLA rumen-unprotected supplement was successful in reducing milk fat content and yield, as well as increasing trans-10, cis-12 CLA secretion in milk fat, while reducing the proportion of FA < 16C in the CLA and CLASA treatments. These results are in agreement with previous observations with lactating ewes(Reference Ticiani, Urio and Ferreira9,Reference Oliveira, Gama and Fernandes13) and goats(Reference Baldin, Gama and Dresch18,Reference Fernandes, Gama and Ribeiro19) .
Dietary supplementation of SA was not able to increase fat content and yield when compared with Control, or it was not able to overcome the antilipogenic effects of trans-10, cis-12 CLA. These observations concur with the results reported by Toral et al. (Reference Toral, Hervás and Carreño4) and Toral et al. (Reference Toral, Hervás and Frutos5), where supplementation of C18:0 did not result in recovery from marine oil-induced MFD regardless of dose. However, Piantoni et al. (Reference Piantoni, Lock and Allen1) reported higher milk fat synthesis in cows supplemented with C18:0 relative to Control (no supplemental fat). The reason explaining the lack of effect of SA supplementation under normal or milk fat-depressing conditions in the present experiment is not clear, as supplemental dietary fat had shown to increase milk fat by providing preformed FA for mammary synthesis of milk TAG(Reference Rabiee, Breinhild and Scott20,Reference Loften, Linn and Drackley21) .
The reduced concentrate intake caused by CLA treatment is consistent with the reduction of nutrients for milk fat synthesis, a non-significant reduced milk yield and a lower BCS (Table 1). The silage intake was reduced by SA compared with Control, and although the mechanism behind such a reduction is not clear, it could be associated with a higher energy density limiting feed intake(Reference Drackley and D’Mello22). The lack of effect of C18:0 on milk fat synthesis observed in this study may be related to its low intestinal digestibility(Reference Piantoni, Lock and Allen1,Reference Allen23) , which would limit its availability for fat synthesis in the mammary gland. SA decreases FA digestibility in the duodenum, with the greatest effect observed for the digestibility of SA itself(Reference Boerman, Firkins and St-Pierre24), what may be related to its low solubility, impairing its incorporation into micelles, thus reducing FA absorption by the small intestine(Reference Drackley and D’Mello22).
Reduced dry matter intake may limit the supply of preformed FA to the mammary gland, and although intake of concentrate was similar between the Control and the SA treatments, silage intake was substantially reduced for SA, thus diluting any potential effects of C18:0 supplementation relative to Control.
The CLA and CLASA treatments reduced FA secretion of C6:0, C8:0, C10:0 and C12:0 as well as ACACAα PII, providing support for the role of CLA in reducing gene expression of lipogenic enzymes and highlighting the lack of effect of C18:0 in overcoming MFD. The observed reduction in ACACAα PII gene expression in CLASA and SA is in agreement with previously published studies(Reference Kadegowda, Bionaz and Piperova25–Reference Suárez-Vega, Gutiérrez-Gil and Toral27). Hansen and Knudsen(Reference Hansen and Knudsen28) and Kadegowda et al. (Reference Kadegowda, Bionaz and Piperova25) outlined possible effects of long-chain SFA on the regulation of ACACAα: (1) C18:0 inhibits de novo synthesis by down-regulating the gene expression of ACACAα; (2) possible competition between long-chain and medium chain acyl-CoA, reallocating FA in the sn-2 and sn-3 positions of the TAG and (3) long-chain acyl-CoA causes a suppressive effect on ACACAα activity. Interestingly, based on the observed inhibitory effects of CLA and C18:0 on ACACAα expression, additive effects could be expected. However, the CLASA treatment did not result in greater inhibition relative to CLA or SA and, on the contrary, resulted in increased ACACAα PII expression in the present study.
In contrast to ACACAα PII, the expression of fatty acid synthase was not affected by SA relative to Control and seemed to counter the effects of CLA in the CLASA treatment. Rico et al. (Reference Rico, Parales and Corl29) reported no effect of abomasal infusions of C18:0 on ACACAα and fatty acid synthase gene expression in dairy cows. Further, the mRNA abundance of PPAR-γ, a key transcription factor for lipogenic genes, was increased by the SA treatment, which suggests that this saturated FA is a natural agonist for PPAR-γ. On the other hand, sterol regulatory element-binding protein 1, also a key regulator of lipogenesis, was not affected by C18:0 as showed in a previous study(Reference Rico, Parales and Corl29).
The SA treatment increased the abundance of CD36 mRNA compared with the Control and CLASA. This protein is associated with the transport of preformed FA into the mammary gland(Reference Bionaz and Loor30). In addition, as described by Glatz et al. (Reference Glatz, Luiken and Bonen31), CD36 has a greater affinity for long-chain FA, especially C18:0, suggesting that it is the main transporter of C18:0 in the mammary gland of lactating sheep. In agreement with our study, Yonezawa et al. (Reference Yonezawa, Yonekura and Kobayashi32) and Kadegowda et al. (Reference Kadegowda, Bionaz and Piperova25) described that the abundance of CD36 mRNA was increased when mammary epithelial cells from dairy cows were cultured with SA. However, this behaviour was not observed with the CLASA treatment, indicating that the trans-10, cis-12 CLA inhibited the abundance of CD36 mRNA, and that C18:0 was not able to overcome this effect.
Lastly, stearoyl-CoA desaturase 1 mRNA abundance was reduced by SA and CLASA treatments relative to Control, suggesting that the FA desaturase capacity may have been compromised with the supplementation of C18:0. Also, the C16:1 and C18:1 desaturase indexes were reduced by the CLASA treatment. A similar reduction in the mammary stearoyl-CoA desaturase 1 gene expression and desaturate index was also observed in dairy cows supplemented with SA, although the mechanism behind this effect was unclear(Reference Rico, Parales and Corl29).
Conclusion
SA supplementation did not increase milk fat synthesis under normal or CLA-induced MFD scenario. CLASA had the same effects as trans-10, cis-12 CLA fed alone, reducing the gene expression of lipogenic enzymes in ewes in a MFD state, showing that SA is not able to reverse CLA-induced MFD by altering gene expression. The CD36 FA translocator is an important long-chain FA transporter in mammary gland and is up-regulated by SA. Overall, increasing provision of SA may not be a viable method to increase milk fat synthesis in dairy ewes.
Acknowledgements
The authors would like to thank all the collaborators at Fazenda Estrela da Serra, especially Mr. Paulo Gregianin, to technicians from UDESC and UFBA and to Dr. Daniel Rico for their assistance and contributions to this study.
This work was funded by the Foundation for the Support of Research and Innovation of the State of Santa Catarina – FAPESC (PAPI UDESC/FAPESC TR584/2019).
The authors’ contributions were as follows: D. E. D. O. designed the research and conducted data analysis. G. C. D. A., D. E. D. O. and C. V. D. M. R. wrote the manuscript. G. C. D. A., R. H. and C. G. P. conducted the animal experiment on the farm. G. C. D. A. and R. H. conducted the analysis of feed, milk composition and gene expression with direction from D. E. D. O. In addition, C. V. D. M. R. conducted an analysis of the profile of fatty acids in milk and contributed to the understanding of the results obtained. All authors approved the final manuscript. D. E. D. O. has primary responsibility for the final content.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S000711452100430X










