Introduction
Glioblastoma is the most common malignant primary brain tumor in adults, with an incidence of 3 to 5 cases per 100,000 population per year.Reference Preusser, de Ribaupierre and Wöhrer 11 The peak incidence of glioblastoma is in the seventh and eighth decades of life, and approximately half of all patients with glioblastoma are older than 65 years. As the incidence of glioblastoma in the elderly population continues to increase, it is expected that the number of glioblastoma patients aged 80 and older (“oldest-old”) 18 will further increase. The median overall survival (OS) of patients with glioblastoma treated with radical adjuvant chemoradiotherapy is 12 to 18 months. However, the best reported outcomes derive from cohorts with a median age of 55-60 years,Reference Stupp, Hegi and Mason 16 , Reference Stupp, Mason and van den Bent 17 while the outcomes remain poorer (i.e., median survival of 8-10 months) in the elderly population, widely defined as older than 60 to 70 years.Reference Perry, Laperriere and O’Callaghan 9 , Reference Rusthoven, Koshy and Sher 14
There is a paucity of data to inform the best management strategy for oldest-old patients with glioblastoma, as patients older than 70 years were excluded from the first EORTC–NCIC trial, and data from the CCTG CE.6 trial in elderly patients showed a median OS of 9.3 months in newly diagnosed glioblastoma (median age of 73 years old, and less than a third were older than 75 years) treated with combined modalities.Reference Perry, Laperriere and O’Callaghan 9 In order to optimize therapeutic approaches in elderly patients with glioblastoma, it is important to quantify treatment outcomes in this specific population, as many are unable to receive or tolerate radical therapy due to frailty, comorbidities, and lack of access to care and/or caregivers. Pivotal randomized trials have only included a small proportion of oldest-old patients with glioblastoma, and detailed data on this unique cohort have not been reported. As cited, the CCTG CE.Reference Laperriere, Weller and Stupp 6 trial reported that approximately 30% of patient were >75 years old, but it is unclear how many, if any, were older than 80 years.Reference Perry, Laperriere and O’Callaghan 9 In this scenario, institutional-based analyses are valuable, as they might convey a more accurate representation of treatment outcomes in this oldest-old population.
We conducted a retrospective review on consecutive patients with glioblastoma aged 80 years or older treated with surgical resection or biopsy followed by best supportive care, TMZ alone, radiotherapy (RT), or a combined-modality approach. Our objective was to report the disease outcomes and treatment toxicities in this unique oldest-old population outside a controlled clinical trial setting.
Methods
After obtaining institutional approval, the prospective brain tumor registry at our institution was used to identify consecutive primary glioblastoma patients older than 80 years referred to our institution for treatment, outside a clinical trial setting, between January of 2004 and November of 2009. We included patients managed with best supportive care (BSC), TMZ alone, radiotherapy alone, or concomitant with TMZ. Before treatment, a multidisciplinary team that included radiation oncologists, neurooncologists, and neurosurgeons determined the best management approach. Clinical demographics (including ECOG–PS and Age-Adjusted Charlson Comorbidity Index [ACC]) were collected (see Supplementary Material 1). The ACC, follow-up information, physical assessment, and repeated magnetic resonance imaging (MRI) were available at baseline and until the time of analysis or the patient’s death.
Surgery, Radiation Therapy, and Chemotherapy
Maximum surgical resection was offered and attempted for all patients unless the patient presented with a performance status [PS] ≥3, critical brain cortex location, multiple or severe comorbidities, or if the patient declined the surgical procedure. Radiotherapy was delivered on a daily basis. Before simulation, all patients were immobilized with a customized thermoplastic mask. Simulation and planning RT studies included computed tomography (CT) and MRI of the brain, which were coregistered. Gross tumor volume (GTV) delineation was based on the surgical cavity (if present) and enhancing lesion defined on T1-wighted imaging with gadolinium. Clinical target volume (CTV) was created by an isotropic expansion of 1.5 cm, trimmed at anatomical boundaries (i.e., skull, tentorium). An additional margin of 0.5 cm was added for planning target volume (PTV). Radiotherapy dose schedules were 20 Gy in 5 fractions (poor PS and frail), 30 Gy in 10 fractions (poor PS and large volume or multifocal disease), and 40 Gy in 15 fractions (good PS and previous subtotal surgical resection [STR] or gross total resection [GTR]). When prescribed, chemotherapy consisted of TMZ (75 mg/m²), once daily, 7 days a week, from the first until the last day of RT. Patient status with respect to O-6-methylguanine DNA methyltransferase (MGMT) promoter methylation and IDH1/2 mutation were not available, because those tests were not routinely performed during the study period outside the clinical trial setting.
Follow-Up
After treatment, clinical follow-up visits with MRI or CT scans were performed every 2-3 months. Acute toxicities (<3 months after treatment) were ascertained using the Common Terminology Criteria for Adverse Events (v. 4.0). Progression was defined as radiologic evidence of local tumor recurrence or distant recurrence outside the previously treated volume or neurologic decline without radiologic confirmation.
Statistical Analysis
Summary statistics were used to describe patient, lesion, and treatment characteristics. ECOG–PS and ACC cutoffs were defined using the median. Age was defined by the clinical focus of this publication. Surgical status: any kind of surgical resection versus biopsy only (clinical judgment). Progression-free survival (PFS) and overall survival (OS) were analyzed from date of diagnosis until the measured event, or censored at the last follow-up date if no event had occurred. Survival curves were generated by the Kaplan–Meier method, and the log–rank test was performed to estimate differences between survival outcomes. All tests were two-sided, and a p value of less than 0.05 was considered significant. All analyses were performed using IBM SPSS Statistical Software (v. 21; SPSS Inc., Chicago, Illinois).
Results
Among 883 patients in the brain tumor database from January of 2004 to November of 2009, a total of 48 patients 80 years old or older (41 were 80-85 years old and 7 were >85 years of age) were identified and included for analysis. Patient, tumor, and clinical characteristics are shown in Table 1. The majority were male 30/48 (62.5%), and the median ECOG-PS at presentation was 2 (0-4). The median ACC at presentation was 6 (4-15): ≤6 in 32 (66.7%) patients. The most common symptom at presentation, tumor location, and laterality were personality or cognitive changes (50%), frontal lobe (35.4%), and left-brain (43.7%), respectively. Tumors were multifocal or bifrontal in 10/48 (20.9%). Midline shift was present in 36/48 (75.0%) of patients, and tumor median maximum diameter was 4.5 cm (range=1.6-9.2 cm). Steroids were prescribed at the time of initial diagnosis in 44/48 (92%) of cases, and the mean dexamethasone dose and duration prior to the first RT visit were 8.4 mg/daily (SD±4.9 mg/daily) and 7 days (SD±3.2 days), respectively.
Table 1 Patients and treatment characteristics
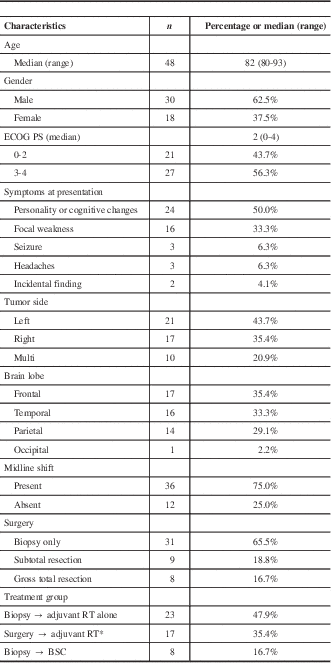
ECOG–PS: Eastern Cooperative Oncology Group–Performance Status; RT=radiotherapy; BSC=best supportive care.
* 3 patients received RT plus concomitant TMZ; 14 received RT alone.
The median time between diagnosis and treatment was 22 days (range=5-45 days). Biopsy only was performed on 31/48 (65.5%) of the patients. Reasons for not undergoing surgical resection were a poor PS ≥ 3 (10/31 patients [32.2%]); multiple or severe comorbidities (8 [25.8%]); multifocal tumor (7 [22.5%]); and patient or power of attorney decision (6 [19.3%]). Of the remaining 17 (35.4%) patients, 9 had STR and 8 underwent GTR. Adjuvant RT alone was administered to 37 patients (77%), no patient received TMZ alone, and 3 (6.2%) received combined RT and TMZ. All three patients in the latter group had PS<2, ACC<6, and GTR. Overall, biopsy followed by RT was delivered to 23 patients, surgery followed by RT ± TMZ to 17 patients, and biopsy followed by BSC to 8 patients.
Disease progression was clinically determined in 19/37 (52%) patients, and both clinically and on imaging in 18/37 (48%) patients. Of the 18 patients with documented imaging progression, 16 (89%) had unifocal in-field recurrence within the PTV. Cause of death was directly attributable to neurological progression in 29/48 (60.4%), to infectious process in 8/48 (16.6%), and not definable in 8/48 (16.6%).
Progression-Free and Overall Survival Analyses
After a median follow-up of 132 days (4.4 months; interquartile range [IQR]=17 days to 31.2 months), the median OS and PFS for the full cohort was 4.1 months (95% CI 3.3-4.9) and 2.7 (95% CI 1.5-3.9) months, respectively. The median OS for surgery followed by RT or RT plus TMZ, biopsy followed by RT, and biopsy followed by BSC was 7.1 (95% CI 2-12.4), 4.2 (95% CI 3.1-5.4), and 2.0 (95% CI 1.5-2.4) months, respectively (p=0.002). Interestingly, the three cases treated with RT+TMZ exhibited OS ranging from 7.8 to 31.2 months. The median PFS was 4.9 (95% CI 4-5.9) months for surgery followed by RT or RT plus TMZ, 2.6 (95% CI 0.8-4.46) months for biopsy followed by RT, and 2.0 (95% CI 0.5-0.66) months for biopsy followed by BSC (p=0.002). In Figure 1, Kaplan–Meier curves for OS and PFS are displayed for each treatment arm. Surgical resection, age≤85, and AAC<6 were associated with better OS (p=0.032, p=0.031, and p=0.02, respectively). Univariate analyses demonstrating predictors of OS are shown in Table 2. In Figure 2 we report the Kaplan–Meier curves for the factors other than age (ACC and surgical resection status) that correlate with improved OS.
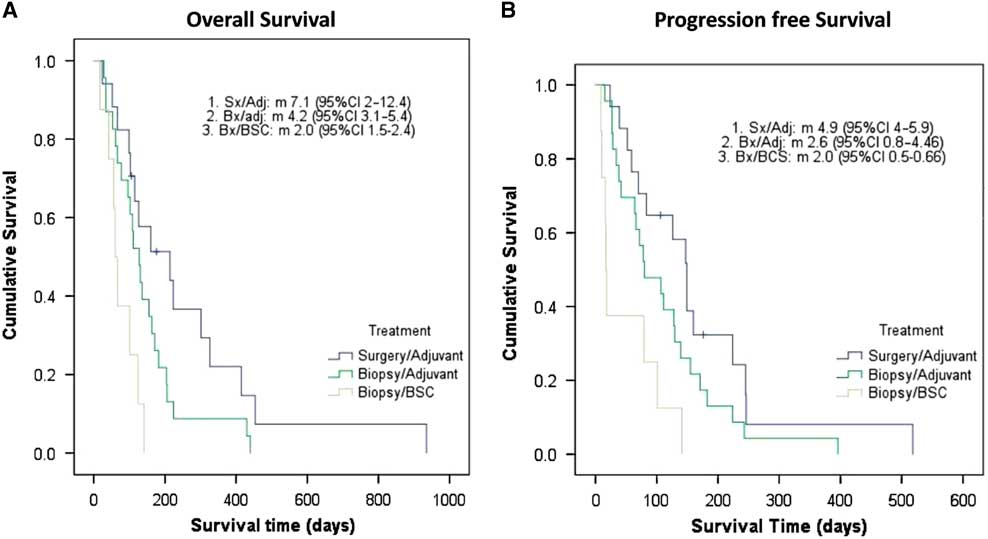
Figure 1 Elderly population with glioblastoma: Kaplan–Meier curves of overall survival (1A) and progression-free survival (1B). Adj=adjuvant treatment (radiotherapy alone or chemoradiation); BSC=best supportive care only; Bx=biopsy only; Sx=surgery.
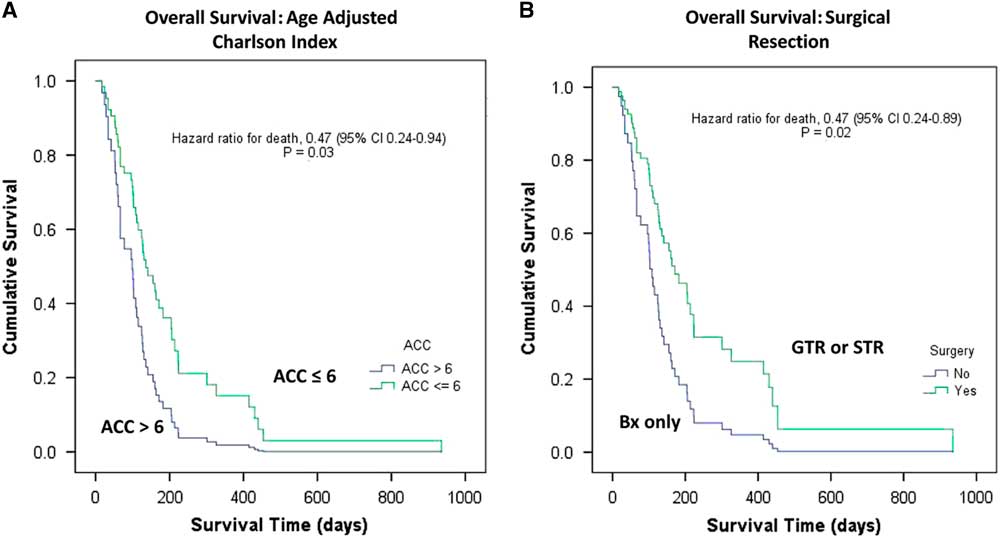
Figure 2 Elderly population with glioblastoma. Univariate Kaplan–Meier curves of overall survival as a function of (2A) ACC≤6 vs. >6; and (2B) surgical resection: surgery versus no surgery. ACC=Age-Adjusted Charlson Index; Bx=biopsy; GTR=gross total resection; STR=subtotal resection.
Table 2 Univariate analyses demonstrating predictors of overall survival
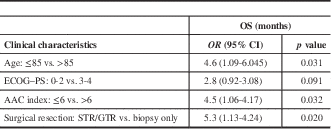
AAC=Age-Adjusted Charlson Index; ECOG–PS=Eastern Cooperative Oncology Group–Performance Status; GTR: gross total resection; OS=overall survival; STR=subtotal resection.
Toxicity
The median RT treatment time was 15.15 days (SD±8.6 days). Three patients required transient treatment interruption due to toxicity or worsening neurological symptoms. The mean interruption time was 5.8 days (SD±3.8 days). However, all patients subsequently completed their full proposed treatment schedule. Median ECOG–PS posttreatment was 2 (range=1-4). Maximum acute toxicity during the first 60 days post RT treatment was grade 0, 1-2, and 3-4 in 12/30 (40.0%), 15 (50.3.%) and 2 (6.7%) patients, respectively.
Considering the potential frailty of this age group, the mortality rates at 30 and 60 days after the start of RT were determined as an additional surrogate for treatment-related toxicity; 3 (6.2%) and 10 (20.8%) patients died within these timeframes, respectively. However, these results were proportionally lower than the same endpoints from time of diagnosis among patients managed with BSC (16.2% and 59.1%, p=0.04 and p=0.036, for 30 and 60 days, respectively).
Discussion
In this single-institution experience of oldest-old patients with glioblastoma, the clinical outcomes (OS and PFS) remained poor, reflecting a need for better management strategies for this specific population. In subgroup analysis, surgical resection, AAC ≤ 6, and age ≤ 85 were associated with significantly longer overall survival. In addition, surgical resection followed by combined adjuvant treatment (RT+TMZ) showed better OS outcomes, though low numbers and inherent selection bias preclude strong conclusions.
Over the previous 20 years, there have been several studies aimed at defining the best treatment approach for glioblastoma in the elderly, including six randomized trials with heterogeneous populations, endpoints, and interventions. However, despite being at the incidence peak and a growing population, the “oldest-old” (>80 years) have been largely underrepresented in these studies. To our knowledge, ours represents the largest series to date addressing treatment outcomes of glioblastoma in the oldest-old, showing the real-world experience of a tertiary cancer center in managing this selected population outside the setting of a highly controlled clinical trial.
Several important findings emerge from our study. We observed a median overall survival of 4.1 months, with the most favorable outcome seen in patients treated with surgical resection followed by RT or RT+TMZ rather than best supportive care (7.1 vs. 2.0 months, p=0.002). In addition, those undergoing surgery (gross or subtotal resection) as part of their treatment showed improved OS and PFS, without an observable increase in treatment related toxicity. All patients who received RT completed their treatment, and toxicities were generally minimal and manageable. Nonetheless, the mortality rates at 30 (6.2%) and 60 days (20.8%) after RT initiation (frequently used in surgical literature to denote treatment-related mortality) seem unexpectedly high, albeit lower compared to those of patients managed with BSC. Reference Schwarze, Brasel and Mosenthal 15
Age≤85 years and ACC≤6 were also correlated with better OS outcomes. Our data show that patient selection is very important in this population, and prognostic instruments (e.g., the ACC) other than the ECOG–PS alone can perform better selection in this frail population with multiple comorbidities.Reference Chang, Yin and Wei 2 , Reference Charlson, Szatrowski, Peterson and Gold 3 We report a trend of an ECOG–PS of 0-2 being correlated to better OS when compared to a PS of 3-4 (p=0.091). We believe that due to lack of power (n=48) this was not statistically significant. However, the ACC helped to identify oldest-old glioblastoma patients with a higher risk of mortality in our study (p=0.02).
Several groups have aimed to address the treatment benefits and outcomes in elderly patients (see Footnote Table 3).Reference Bracci, Laigle-Donadey and Hitchcock 1 , Reference Keime-Guibert, Chinot and Taillandier 5 , Reference Malmström, Grønberg and Marosi 7 , Reference Perry, Laperriere and O’Callaghan 9 , Reference Piccirilli, Bistazzoni and Gagliardi 10 , Reference Roa, Brasher and Bauman 12 , Reference Roa, Kepka and Kumar 13 , Reference Wick, Platten and Meisner 19 As shown, the definition of elderly varies widely, and the “oldest-old” subgroup (i.e., > 80 years old) has been underrepresented and not been described separately in several randomized trials. With the obvious limitations of interstudy comparisons, the overall survival results of patients treated with surgical resection followed by adjuvant RT ± TMZ in the present cohort suggest that the results of the randomized trials might not be representative for this unique population. In fact, within the NORDIC trial, patients aged >70 years exhibited shorter median survival than those in the range of 60 to 70 years, either when treated with standard (5.2 vs. 7.6 months) or hypofractionated (7 vs. 8.8 months) RT, respectively.Reference Malmström, Grønberg and Marosi 7 These OS differences are unlikely to be explained and/or biased by competing risk factors or differential life expectancy in the context of a highly lethal disease such as glioblastoma.
Table 3 Clinical trials and case series of elderly patients with glioblastoma
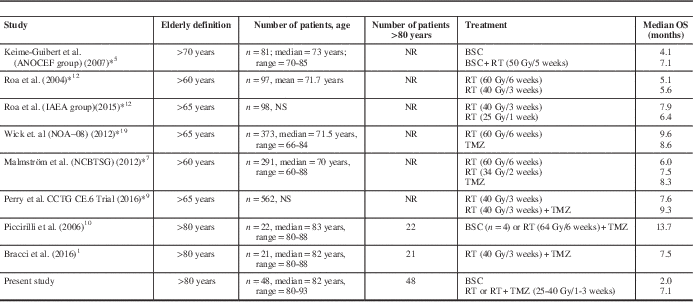
BSC=best supportive care; Gy=gray; NR=not reported; NS=nonsignificant; OS: overall survival; RT=radiotherapy; TMZ=temozolamide.
* Randomized controlled trial.
To our knowledge, only two other series have addressed the issue of survival in the “oldest-old” (>80 years old) glioblastoma patients exclusively. Piccirilli et al.Reference Piccirilli, Bistazzoni and Gagliardi 10 reported the outcomes of 22 oldest-old patients (80-89 years of age) with glioblastoma, of whom 16 (72.7%) were treated with a multimodal radical approach (surgery, radiotherapy, and chemotherapy). As opposed to our cohort, radiotherapy was given with conventional fractionation to a total dose of 64 Gy. Remarkably, multimodal treatment was associated with a mean overall survival of 16.7 months, as opposed to 5.8 months in those treated with either RT or chemotherapy alone after biopsy. Bracci et al.Reference Bracci, Laigle-Donadey and Hitchcock 1 more recently reported a series of 21 patients 80 years and older with glioblastoma in which they aimed to assess the efficacy and safety of hypofractionated RT±TMZ treatment. After approximately 6 months of follow-up, median progression-free and overall survival were 5.8 and 7.5 months, respectively. Hospitalization was required in a third of patients due to toxicity. Taken together and acknowledging selection bias, active treatment appears to confer survival advantages in the oldest-old, although the absolute benefit seems to be less than in younger elderly patients. All this evidence reinforces the need for better clinical assessment strategies for the most elderly, and studies to substantiate individualized therapeutic decisions for an age group that has been poorly studied.
The current study has several limitations inherent to its retrospective nature. Despite being the largest series to date, our absolute numbers are small, rendering this study potentially insufficiently powered to observe differences in progression-free rate in those patients undergoing surgical resection as part of their treatments or when comparing radiation approaches (RT dose and fractionation vary among patients, and we believe that outcomes might differ from one treatment arm to another). Second, comparisons between treatment approaches (i.e., RT vs. BSC), while reassuring, might be a reflection of unaccounted-for bias in patients’ treatment selection. Third, only 3 (7.1%) patients received what today might be considered the current standard of care (i.e., combined RT and TMZ) for the elderly, and they appeared to exhibit better overall survival compared to the median of the cohort.Reference Perry, Laperriere and O’Callaghan 9 Nonetheless, the therapeutic value of combined modality treatment in the “oldest-old” remains unknown because these patients were highly underrepresented (if represented at all) in the pivotal randomized trials. However, given the unique challenges of this age group and the lack of randomized evidence in this population, we believe that this series adds valuable information to the literature that obviously needs to be contextualized when applied to individual cases. Readers must be careful when interpreting these data, as our study population includes patients managed between 2004 and 2009. This may not apply to patients treated now with the technologies currently available.
Moreover, considering the 20.8% mortality rate at 60 days post RT-only treatment, our results suggest that caution should be exercised when extrapolating results of more aggressive treatment approaches to this unique population. Lastly, information on such important molecular prognostic factors as tumor MGMT methylation and IDH1 mutation status were not available during the period of our study. Both markers have been shown to have significant prognostic and predictive value in patients with glioblastoma receiving TMZ alone (no patient received TMZ alone in our study) or in combination with RT.Reference Hegi, Diserens and Gorlia 4 , Reference Nobusawa, Watanabe, Kleihues and Ohgaki 8 , Reference Yan, Parsons and Jin 20 , Reference Yin, Cai and Dong 21 However, the value of these markers in this uniquely understudied population requires further study and independent validation. Many centers do not have access to these markers either at the time of making a treatment decision or at anytime, in which case treatment recommendation would be based on clinical findings and institutional expertise, as was the case in our study.
In summary, our study suggests that surgical resection followed by RT or RT+TMZ is associated with the best OS in oldest-old patients with glioblastoma, albeit limited by potential patient selection bias. Considerable mortality within 30 to 60 days of treatment was observed in a proportion of patients, suggesting a narrow therapeutic index for this combined modality treatment approach.
Conclusions
According to our results, which include the inherent bias of a retrospective review and lack of MGMT methylation status, outcomes in the oldest-old patient with glioblastoma remain poor in terms of both progression-free and overall survival. In a very selected population, we observed superior survival outcomes in patients receiving radical treatment (surgery followed by RT or RT+TMZ), with acceptable but not negligible toxicity rates. In light of the very poor prognosis and frailty in this patient population, selection of patients for aggressive management must be considered very carefully. Further studies focusing on oldest-old glioblastoma patients are warranted to better define the optimum therapeutic strategy for this growing and understudied population.
Acknowledgments
This work was supported by the Gerry and Nancy Pencer Brain Tumor Center.
Disclosures
Dr. Berlin reports nonfinancial support from The Gerry and Nancy Pencer Brain Tumor Center, during the conduct of the study.
Fabio Y. Moraes, Andrea Lo, Erin R. Morgan, Barbara-Ann Millar, David B. Shultz, Catherine Maurice, Craig Harlos, Paul Kongkham, Mark Bernstein, Gelareh Zadeh, Normand Laperriere, and Warren Mason have nothing to disclose.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2017.278.









