Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Aagaard, P.
and
Jahren, J. S.
1992.
Diagenetic Illite-Chlorite Assemblages in Arenites. II. Thermodynamic Relations.
Clays and Clay Minerals,
Vol. 40,
Issue. 5,
p.
547.
de Caritat, Patrice
Hutcheon, Ian
and
Walshe, John L.
1993.
Chlorite Geothermometry: A Review.
Clays and Clay Minerals,
Vol. 41,
Issue. 2,
p.
219.
Hillier, S.
1993.
Origin, Diagenesis, and Mineralogy of Chlorite Minerals in Devonian Lacustrine Mudrocks, Orcadian Basin, Scotland.
Clays and Clay Minerals,
Vol. 41,
Issue. 2,
p.
240.
Jiang, Wei-Teh
Peacor, Donald R.
and
Buseck, Peter R.
1994.
Chlorite Geothermometry?—Contamination and Apparent Octahedral Vacancies.
Clays and Clay Minerals,
Vol. 42,
Issue. 5,
p.
593.
Hillier, S.
1994.
Pore-lining chlorites in siliciclastic reservoir sandstones: electron microprobe, SEM and XRD data, and implications for their origin.
Clay Minerals,
Vol. 29,
Issue. 4,
p.
665.
Barrenechea, J. F.
Rodas, M.
and
Mas, J. R.
1995.
Clay mineral variations associated with diagenesis and low-grade metamorphism of Early Cretaceous sediments in the Cameros Basin, Spain.
Clay Minerals,
Vol. 30,
Issue. 2,
p.
119.
Martínez-Serrano, Raymundo G.
and
Dubois, Michel
1998.
Chemical Variations in Chlorite at the Los Humeros Geothermal System, Mexico.
Clays and Clay Minerals,
Vol. 46,
Issue. 6,
p.
615.
Zane, Antonella
and
Weiss, Zdenek
1998.
A procedure for classifying rock-forming chlorites based on microprobe data.
Rendiconti Lincei,
Vol. 9,
Issue. 1,
p.
51.
Dejou, Jean
De Kimpe, Christian
Macaire, Jean-Jacques
and
Perruchot, Alain
1999.
Chemical Changes and Genesis of Secondary Minerals During the Alteration of Biotites from Ignimbrites in the Tazzeka Mountain (Morocco).
Clays and Clay Minerals,
Vol. 47,
Issue. 2,
p.
144.
Mata, M. P.
Giorgetti, G.
Árkai, P.
and
Peacor, D. R.
2001.
Comparison of Evolution of Trioctahedral Chlorite/Berthierine/Smectite in Coeval Metabasites and Metapelites from Diagenetic to Epizonal Grades.
Clays and Clay Minerals,
Vol. 49,
Issue. 4,
p.
318.
Lougear, André
König, Iris
Trautwein, Alfred X.
and
Suess, Erwin
2001.
Mössbauer investigations to characterize Fe lattice sites in sheet silicates and Peru Basin deep-sea sediments.
Deep Sea Research Part II: Topical Studies in Oceanography,
Vol. 48,
Issue. 17-18,
p.
3701.
Soriano, M.
Sánchez‐Marañón, M.
Melgosa, M.
Gámiz, E.
and
Delgado, R.
2002.
Influence of chemical and mineralogical composition on color for commercial talcs.
Color Research & Application,
Vol. 27,
Issue. 6,
p.
430.
Aja, Stephen U.
2002.
The Stability of Fe-Mg Chlorites in Hydrothermal Solutions: Ii. Thermodynamic Properties.
Clays and Clay Minerals,
Vol. 50,
Issue. 5,
p.
591.
Durand, Claudine
Rebours, Bernadette
Rosenberg, Elisabeth
and
Brosse, Etienne
2003.
2001. A Clay Odyssey.
p.
261.
Ferrage, E.
Martin, F.
Micoud, P.
Petit, S.
De parseval, P.
Beziat, D.
and
Ferret, J .
2003.
Cation site distribution in clinochlores: a NIR approach.
Clay Minerals,
Vol. 38,
Issue. 3,
p.
329.
Ramı́rez-Sánchez, Elisa
Hervé, Francisco
Kelm, Ursula
and
Sassi, Raffaele
2005.
P–T conditions of metapelites from metamorphic complexes in Aysen, Chile.
Journal of South American Earth Sciences,
Vol. 19,
Issue. 3,
p.
373.
Alonso-Azcárate, J.
Rodas, M.
Barrenechea, J. F.
and
Mas, J. R.
2005.
Clay minerals as provenance indicators in continental lacustrine sequences: the Leza Formation, early Cretaceous, Cameros Basin, northern Spain.
Clay Minerals,
Vol. 40,
Issue. 1,
p.
79.
Mücke, Arno
2006.
Chamosite, siderite and the environmental conditions of their formation in chamosite-type Phanerozoic ooidal ironstones.
Ore Geology Reviews,
Vol. 28,
Issue. 2,
p.
235.
Brigatti, M.F.
Galan, E.
and
Theng, B.K.G.
2006.
Handbook of Clay Science.
Vol. 1,
Issue. ,
p.
19.
Timón, S. M.
Moro, M. C.
Cembranos, M. L.
Fernández, A.
and
Crespo, J. L.
2007.
Contact metamorphism in the Los Santos W skarn (NW Spain).
Mineralogy and Petrology,
Vol. 90,
Issue. 1-2,
p.
109.
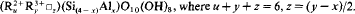 This is superior to the known formulae for the trioctahedral and dioctahedral chlorites. It has been shown that the chlorite end-member compositions of Bayliss (1975) project in one point of the projection field leaving many end-member compositions unnamed. As they correspond to the names used previously, a proposal is made to restore these names.
This is superior to the known formulae for the trioctahedral and dioctahedral chlorites. It has been shown that the chlorite end-member compositions of Bayliss (1975) project in one point of the projection field leaving many end-member compositions unnamed. As they correspond to the names used previously, a proposal is made to restore these names.



