Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Tsuji, Masamichi
Mao, Gang
Yoshida, Takashi
and
Tamaura, Yutaka
1993.
Hydrotalcites with an extended Al3+-substitution: Synthesis, simultaneous TG-DTA-MS study, and their CO2 adsorption behaviors.
Journal of Materials Research,
Vol. 8,
Issue. 5,
p.
1137.
Carlino, Simon
and
Hudson, Michael J.
1995.
Thermal intercalation of layered double hydroxides: capric acid into an Mg–Al LDH.
J. Mater. Chem.,
Vol. 5,
Issue. 9,
p.
1433.
Szostak, R.
and
Ingram, C.
1995.
Catalysis by Microporous Materials, Proceedings of ZEOCAT '95.
Vol. 94,
Issue. ,
p.
13.
Titulaer, M. K.
Talsma, H.
Jansen, J. B. H.
and
Geus, J. W.
1996.
The formation of ice between hydrotalcite particles measured by thermoporometry.
Clay Minerals,
Vol. 31,
Issue. 2,
p.
263.
Ramos, E.
Lopez, T.
Bosch, P.
Asomoza, M.
and
Gomez, R.
1997.
Thermal stability of sol-gel hydrotalcites.
Journal of Sol-Gel Science and Technology,
Vol. 8,
Issue. 1-3,
p.
437.
Fetter, G.
Ramos, E.
Olguin, M. T.
Bosch, P.
López, T.
and
Bulbulian, S.
1997.
Sorption of131I− by hydrotalcites.
Journal of Radioanalytical and Nuclear Chemistry,
Vol. 221,
Issue. 1-2,
p.
63.
Kukkadapu, Ravi K.
Witkowski, Marc S.
and
Amonette, James E.
1997.
Synthesis of a Low-Carbonate High-Charge Hydrotalcite-like Compound at Ambient Pressure and Atmosphere.
Chemistry of Materials,
Vol. 9,
Issue. 2,
p.
417.
Olguín, María Teresa
Bosch, Pedro
Acosta, Dwight
and
Bulbulian, Silvia
1998.
131I− Sorption by Thermally Treated Hydrotalcites.
Clays and Clay Minerals,
Vol. 46,
Issue. 5,
p.
567.
Alcaraz, J
1998.
Solid base catalysts for mercaptan oxidation.
Catalysis Today,
Vol. 43,
Issue. 1-2,
p.
89.
Goswamee, Rajib Lochan
Sengupta, Pinaki
Bhattacharyya, Krishna Gopal
and
Dutta, Dipak Kumar
1998.
Adsorption of Cr(VI) in layered double hydroxides.
Applied Clay Science,
Vol. 13,
Issue. 1,
p.
21.
Elm’chaouri, Abdellah
and
Simonot-Grange, Marie-Hélène
1999.
Données expérimentales et modélisation d’adsorption des systèmes N2(g)/montmorillonite potassique de Camp-Berteau et N2(g)/hydrotalcite carbonatée.
Thermochimica Acta,
Vol. 339,
Issue. 1-2,
p.
117.
Rives, Vicente
and
Angeles Ulibarri, Marı́a
1999.
Layered double hydroxides (LDH) intercalated with metal coordination compounds and oxometalates.
Coordination Chemistry Reviews,
Vol. 181,
Issue. 1,
p.
61.
Serrano, J.
Bertin, V.
and
Bulbulian, S.
2000.
99Mo Sorption by Thermally Treated Hydrotalcites.
Langmuir,
Vol. 16,
Issue. 7,
p.
3355.
Serrano, J.
Granados, F.
Bertin, V.
and
Bulbulian, S.
2002.
Speciation of some235U fission products in nitrate solution and their sorption behavior on thermally treated hydrotalcites.
Separation Science and Technology,
Vol. 37,
Issue. 2,
p.
329.
Mazeina, L.
Curtius, H.
and
Fachinger, J .
2003.
Formation of hydrotalcite-like compounds during corrosion experiments on MTR-FE-Al cladding.
Clay Minerals,
Vol. 38,
Issue. 1,
p.
35.
Stanimirova, Tsveta
and
Kirov, Georgi
2003.
Cation composition during recrystallization of layered double hydroxides from mixed (Mg, Al) oxides.
Applied Clay Science,
Vol. 22,
Issue. 6,
p.
295.
Mazeina, L
Curtius, H
Fachinger, J
and
Odoj, R
2003.
Characterisation of secondary products of uranium–aluminium material test reactor fuel element corrosion in repository-relevant brine.
Journal of Nuclear Materials,
Vol. 323,
Issue. 1,
p.
1.
Das, N.N
Konar, J
Mohanta, M.K
and
Srivastava, S.C
2004.
Adsorption of Cr(VI) and Se(IV) from their aqueous solutions onto Zr4+-substituted ZnAl/MgAl-layered double hydroxides: effect of Zr4+ substitution in the layer.
Journal of Colloid and Interface Science,
Vol. 270,
Issue. 1,
p.
1.
Cantrell, David G.
Gillie, Lisa J.
Lee, Adam F.
and
Wilson, Karen
2005.
Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis.
Applied Catalysis A: General,
Vol. 287,
Issue. 2,
p.
183.
Greenwell, H. Chris
Jones, William
Stamires, Dennis N.
O'Connor, Paul
and
Brady, Michael F.
2006.
A one-pot synthesis of hybrid organo-layered double hydroxide catalyst precursors.
Green Chemistry,
Vol. 8,
Issue. 12,
p.
1067.
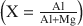 for most of the products is about 0.35, which is at the maximum experimentally-observed limit of solid solubility. Pillared hydrotalcites were also prepared by molybdate, Chromate, and silicate anion replacement. A maximum distance of 10.4 Å between the brucite-like layers was observed for the Mo7O246− intercalated material.
for most of the products is about 0.35, which is at the maximum experimentally-observed limit of solid solubility. Pillared hydrotalcites were also prepared by molybdate, Chromate, and silicate anion replacement. A maximum distance of 10.4 Å between the brucite-like layers was observed for the Mo7O246− intercalated material.



