INTRODUCTION
Gonorrhoea is one of the most prevalent, yet curable, sexually transmitted infections (STIs). Its incidence has increased substantially in many industrialized countries since the mid 1990s, posing a serious public health problem [Reference Browning, Blackwell and Joynson1, Reference Fennema, Cairo and Coutinho2]. In Europe, 50 341 cases were reported in 2012, a rate of 13/100 000 inhabitants [3]. Transmission categories were reported as heterosexual (57%), men who have sex with men (MSM) (38%), with 4% unknown; cases diagnosed in MSM accounted for 69% (n = 15 024) of all male cases [3]. In Catalonia, Spain, 1555 cases of gonorrhoea were reported (21·3/100 000) and of these 46% were MSM, 24% heterosexual men, and 15% women [Reference Casabona4]. MSM are therefore a proven high-risk group towards which it is important to act, through the establishment of control programmes based on effective prevention campaigns and adequate systems for contact tracing and notification.
Molecular typing techniques have been shown to be very useful in clinical epidemiological studies in the field of STIs for the identification of individuals within sexual networks and when combined with epidemiological data, can provide better insights into gonococcal transmission patterns, and aid intervention strategies [Reference Heymans5]. Currently, the Neisseria gonorrhoeae multi-antigen sequence typing (NG-MAST) system is one of the most widely used genotyping methods, as it is a robust technique, easy to carry out, with access to a global database (http://www.ng-mast.net), and has demonstrated a high discriminatory power for identifying individuals in the same sexual network [Reference Martin6, Reference Bilek7].
To our knowledge, there have been relatively few studies investigating gonococcal transmission networks within MSM populations [Reference Heymans5, Reference Choudhury8, Reference Howie9]. Moreover, none of these studies compared the antimicrobial susceptibility of N. gonorrhoeae between the MSM and heterosexual populations together with highly discriminatory molecular typing. We therefore set out to identify possible transmission patterns in individuals through a comparison of the antimicrobial susceptibility and genogroups of gonococci isolated from MSM and heterosexual patients.
METHODS
Study population
From January to December 2013, a total of 339 isolates of N. gonorrhoeae were gathered from 321 patients attending the Drassanes STI Unit in Vall d'Hebron Hospital, Barcelona, Spain. This is the sole STI clinic in the city and offers anonymous and free-of-charge testing and treatment to nearly 10 000 patients a year.
Of the 321 patients, all samples from heterosexuals (n = 54) were included, and 53 from randomly selected MSM. From these 107 patients, 114 isolates were collected but three isolates were excluded from the study as they proved to be the same strain as was isolated from two different anatomical sites (pharynx and rectum in two patients, and pharynx and cervix in one). Of the 111 strains included in the final dataset, three were from a single patient who had presented with three different episodes of gonococcal infection separated by 2 and 3 months; another two strains with distinct sequence types (STs) were from a patient who had had two infection episodes only 10 days apart; and finally, two strains with distinct STs were from two separate episodes, 3 months apart. Of these 111 strains, 57 were from the 54 heterosexuals and 54 from the 53 MSM patients.
Data collection
Demographic, clinical and behavioural data were obtained in the clinic and recorded on standardized forms, including sex, age, sexual orientation, country of birth, HIV status, site of infection, symptomatology, concurrent STIs and numbers of partners in the previous 2 months.
Culture and antibiotic susceptibility
Urethral, endocervical, pharyngeal and/or rectal specimens were collected in Amies broth (Deltalab, Spain) and cultured on selective Thayer–Martin medium incubated for 24–48 h at 35–37 °C in a 5% CO2 atmosphere. Suspected N. gonorrhoeae colonies were identified by Gram stain, the cytochrome oxidase test, and mass spectrometry (MALDI-TOF, Vitek MS system, bioMérieux, Spain).
The minimum inhibitory concentrations (MICs) of penicillin, ceftriaxone, cefixime, azithromycin, ciprofloxacin and spectinomycin were determined by the Etest method (bioMérieux, Spain), as specified by the manufacturer. N. gonorrhoeae ATCC 49 226, for which the MICs of the antimicrobial agents tested were known, was used as the reference strain. MICs were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [10]. Penicillinase production was evaluated using nitrocefin disks. Strains were frozen at −80 °C in trypticase soy broth with 20% glycerol for molecular studies.
DNA extraction and molecular typing
DNA was extracted as follows: a colony from 24 h incubation was re-suspended in 100 µl Milli-Q water, heated at 95 °C for 10 min, centrifuged at 13 000 rpm for 3 min, and the supernatant was diluted 1:2 with Milli-Q water.
Molecular typing was performed using the NG-MAST protocol as described in www.ng-mast.net. Briefly, after DNA extraction, internal fragments of the porB and tbpB genes were PCR-amplified and sequenced in both directions. Each allele was assigned a number, and the combination of the two alleles from each strain was assigned a ST number. Closely related STs were clustered in genogroups (G), as described by Chisholm et al. [Reference Chisholm11], such that each genogroup included all STs that shared one allele and showed >99% similarity with the other allele (⩽5 bp difference for porB and ⩽4 bp for tbpB).
Statistical analysis
Possible differences between MSM and heterosexual groups were compared using the χ 2 test for categorical variables and Mann–Whitney U test for continuous variables. P < 0·05 defined statistically significant difference.
RESULTS
The demographic and epidemiological data for the 107 study patients are summarized in Table 1. Of these patients 90·7% were men. The majority (47·7%), particularly MSM subjects, originated from European countries. Most patients were symptomatic at the time of the consultation. A minority (17·8%) of all subjects was HIV positive with a significantly higher prevalence in MSM than in the heterosexual group (P < 0·001). Approximately one-quarter (24·2%) of subjects had had an STI in the previous 12 months, the most prevalent being gonococcal infection (12·1%), followed by syphilis (4·7%), Chlamydia trachomatis infection (3·7%), and lymphogranuloma venereum (3·7%).
Table 1. Demographic, behavioural and clinical characteristics of patients with gonorrhoea
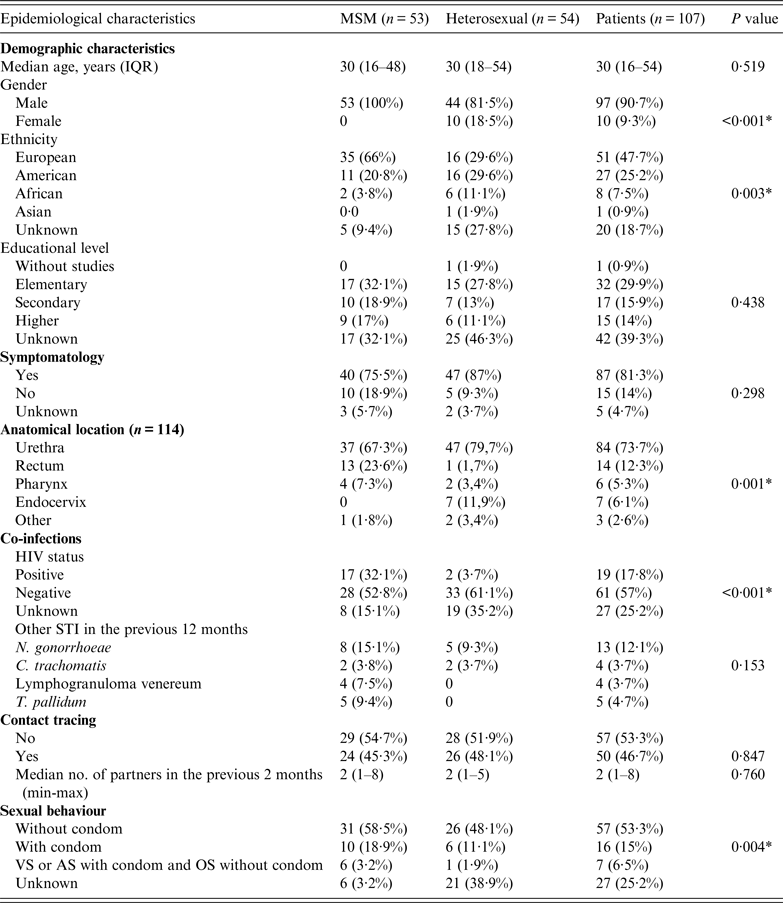
MSM, Men who have sex with men; IQR, interquartile range; VS, vaginal sex; AS, anal sex; OS, oral sex.
* P < 0·05.
Antimicrobial susceptibility
The antimicrobial susceptibility of the strains from patients with different sexual behaviours is detailed in Figure 1. Of the 57 strains from heterosexuals, 13·2% were penicillinase producers. One strain (1·8%) exhibited resistance to ceftriaxone (MIC 0·125 mg/l) and 11 (19·3%) to cefixime (MIC >0·125 mg/l). Nine strains (15·8%) showed reduced susceptibility (MIC 0·25–0·5 mg/l) or full resistance (MIC >0·5 mg/l) to azithromycin, and most (70·2%) were resistant to ciprofloxacin (MIC >0·06 mg/l). in the 54 strains from MSM patients, 13·5% were penicillinase producing but none was resistant to ceftriaxone and only two (3·7%) showed resistance to cefixime. Four strains (7·6%) exhibited intermediate susceptibility or resistance to azithromycin, and 39·6% of all strains were resistant to ciprofloxacin.

Fig. 1. Antimicrobial resistance of N. gonorhoeae isolated from patients with different sexual behaviours. * Statistically significant differences between heterosexual and MSM groups of patients. PPNG, Penicillinase-producing N. gonorrhoeae; I, intermediate susceptibility; R, resistance; HTS, heterosexual; MSM, men who have sex with men. Clinical resistance breakpoints (MIC) according to European Committee on Antimicrobial Susceptibility Testing: ceftriaxone >0·125 µg/ml; cefixime >0·125 µg/ml; azithromycin I >0. 25 µg/ml and R >0·5 µg/ml; ciprofloxacin 0·06 µg/ml.
Although in general the rates of resistance to the antimicrobials studied were higher in the strains from heterosexual than in MSM patients, only resistance to cefixime (P = 0·0159) and ciprofloxacin (P = 0·002) reached statistical significance.
Genotyping
Sixty-two different STs, 15 of which were novel, were identified in the 111 strains. The most frequent was ST2400 (n = 9 strains), followed by ST2992 (n = 7) and ST5793 (n = 6). All ST2400 strains originated from MSM patients, were intermediate susceptible to penicillin but fully susceptible to ceftriaxone and azithromycin; one ST2400 strain was resistant to cefixime and all others were resistant to ciprofloxacin. Five of the seven ST2992 strains were isolated from MSM patients and two from heterosexuals. All were susceptible to the antibiotics tested, except for two which showed intermediate susceptibility to penicillin. All ST5793 strains were isolated from MSM patients and were susceptible to ceftriaxone, cefixime, azithromycin, ciprofloxacin and spectinomycin, but resistant or with intermediate susceptibility to penicillin.
Twenty-one of the 62 STs were grouped into six genogroups, the most prevalent being G1407 (16 strains, 14·4%), G2992 and G2400 (10 strains each, 9%), G1034 (7, 6·3%), G190 (6, 5·4%), and G225 (4, 3·6%). It was not possible to assign ST5793 to a genogroup.
Table 2 shows the distribution of the STs and genogroups of strains according to the sexual orientation of the patients. The most heterogeneous strain group was that from heterosexual patients with 37 different STs, of which 14 fell into five genogroups; the most prevalent genogroup in these patients was G1407 (26·3%), followed by G190 (8·8%), G225 (7%), G1034 (3·5%) and G2992 (3·5%). Thirty different STs were found in MSM patients, with 11 assigned to five genogroups, which in order of prevalence were G2400 (18·5%), G2992 (14·8%), G1034 (9·3%), G190 (1·9%) and G1407 (1·9%).
Table 2. Distribution of sequence types (STs) and genogroups of N. gonorrhoeae according to sexual orientation of patients
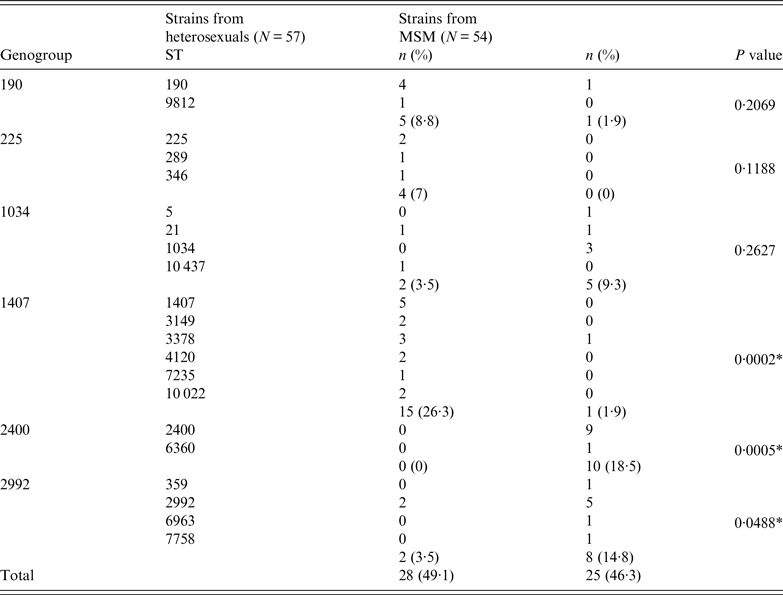
* Statistically significant difference (P < 0·05).
A statistically significant relationship was observed between MSM and genogroups G2400 (P = 0·0005) and G2992 (P = 0·0488), and between heterosexual patients and genogroup G1407 (P = 0·0002), while genogroups G190, G225 and G1034 appeared to be randomly distributed between these two groups.
DISCUSSION
The identification and characterization of sexual networks as well as transmission patterns, could be useful in designing intervention strategies directed at the highest risk population that contributes most to the spread of gonococcal infection, which is the MSM group [3, Reference Casabona4].
This study analysed the differences in antimicrobial susceptibility and the epidemiological type distribution of 111 strains of N. gonorrhoeae from patients with different sexual orientation. Some significant differences were found between the population groups in that heterosexual patients were most often infected with gonococcal strains that were more resistant to ciprofloxacin and cefixime than MSM patients. Moreover, we have demonstrated the circulation of different gonococcal strains in the two groups as MSM were more likely to become infected with strains of genogroups G2992 and G2400, compared to heterosexuals, who were more likely to be infected with genogroup G1407 strains.
Genogroup G1407 appears to be the most prevalent in Europe [Reference Chisholm11] having been associated with cases of therapeutic failure in France [Reference Unemo12], Spain [Reference Camara13], Slovenia [Reference Unemo14], Norway [Reference Unemo15], Austria [Reference Unemo16] and the UK [Reference Ison17]. The fact that in our study G1407 was the most prevalent in the heterosexual population, which at the same time were infected by more resistant strains, reinforces our previous findings [Reference Serra-Pladevall18], in which G1407 was clearly related with decreased susceptibility to the extended spectrum cephalosporins, and G2992 with susceptible strains. This could explain why the strains infecting heterosexual patients in our area are more resistant than those infecting MSM, likely because cluster G1407 is circulating in the heterosexual population and it is known that this genogroup develops resistance with greater frequency [Reference Serra-Pladevall18].
Few studies in Europe have explored the distribution of N. gonorrhoeae strains in populations with different sexual orientations [Reference Heymans5, Reference Choudhury8, Reference Howie9, Reference Kolader19]; the majority of these were done in the UK [Reference Choudhury8] and Holland [Reference Heymans5, Reference Kolader19, Reference Van20], but to date not in Spain.
Choudhury et al. in 2004 [Reference Choudhury8], used NG-MAST to show the circulation in London of distinct strains of gonococci between the sexual networks of MSM and heterosexuals. They described 21 clusters (STs described in ⩾20 patients) of which, seven were almost exclusively from male patients (⩾77% MSM), with ST225 and ST359 predominant. Most (94%) of the 14 remaining clusters were from heterosexuals. In our study, ST359 is included within G2992, and showed a statistically significant relationship with the MSM population. Nevertheless, and in contrast to Choudhury et al. [Reference Choudhury8], we found G225 to be more frequent in heterosexuals.
The results of our study are not easily comparable with those performed in Holland [Reference Kolader19, Reference Van20] owing to the use of different typing techniques. Nevertheless, Van Duynhoven et al. [Reference Van20] showed by a serotype analysis of gonococci isolated in 1994 that strain populations varied according to sexual behavioural group with serotypes IB2, IB6 and IA5 being more prevalent in MSM in contrast to serotypes IB1 and IB3 in heterosexuals. Similarly, Kolader et al. [Reference Kolader19], in a study done between 2002 and 2003 using typing of por and opa genes, described 11 major clusters (with ⩾20 patients with the same por-opa pattern), seven of which came predominantly from MSM patients, three were almost exclusively from heterosexual patients, and one mixed cluster (46% MSM, 54% heterosexuals).
Finally, Chisholm et al. [Reference Chisholm11] used NG-MAST to genotype 1066 gonococcal isolates from the European Gonococcal Antimicrobial Surveillance Programme, recovered in 2009 and 2010 in 21 European countries, and identified 406 different STs. A review of the more frequent STs in the survey showed that while ST1407 was the most prevalent, on both a European level and in the 100 strains included from Spain, in this study this ST was the fourth most frequent. ST2992 was the second most common in both studies, and the third, ST225, was identified in only two strains here. By contrast, ST2400, the most prevalent in our study (8·3%), accounted for only 0·7% of the European series. Major differences between the two studies are evident in the prevalence of genogroups, although both found G1407 to be predominant. Moreover, both studies observed a statistically significant relationship between MSM and genogroup G2992, but in our study G1407, was more associated with heterosexuals than with MSM, as found by Chisholm et al. [Reference Chisholm11].
These findings highlight the existence of major differences in the geographical distribution of strain populations of N. gonorrhoeae at both the global and local level. This makes it impossible to extrapolate results, and emphasizes the importance of performing local studies. As a consequence, we consider it would be useful locally to monitor antimicrobial resistances in strains from different populations in order to keep treatment guidelines updated and, if important differences emerge between populations, empirical treatment of patients could be changed accordingly.
In order to control and detect new transmission patterns, prospective strain typing studies using NG-MAST would allow us to distinguish those types that have long been present in the population, representing endemic transmission, from novel strain clusters which might indicate local outbreaks, so as to inform public-health interventions. The rapid identification of such new strains spreading in patient subpopulations not only allows for speedy immediate intervention, but also improves the chances to prevent these strains becoming irreversibly established in a patient group. In this context, it is noteworthy that our study revealed a strong association between infection with G2400 strains and MSM, which to our knowledge has not previously been described.
A limitation of our study is that the population surveyed consisted only of patients attended an STI clinic and as a result it is not a random sample of the general population, even though the study clinic serves the majority of the MSM population in our area.
In conclusion, we have documented a high prevalence of genogroups G1407, G2992 and G2400 in the Barcelona area and highlighted significant differences in antimicrobial resistance patterns and the distribution of different gonococcal populations in patients with distinct sexual practices. The gonococci that infect heterosexual patients are more resistant to ciprofloxacin and cefixime and associated with genogroup G1407, while MSM are more likely to become infected with more susceptible strains from genogroups G2992 and G2400.
ACKNOWLEDGEMENTS
This study was funded by a grant (PI12/01 808) from the Instituto de Salud Carlos III, and co-financed by the European Regional Development Fund. The authors thank Nieves Larrosa, Juan José González and Oihane Irazoki for their contributions and comments in the realization of this work.
DECLARATION OF INTEREST
None.







