INTRODUCTION
The food chain represents a major route of transmission of animal infections to humans. Between 1992 and 1999 it was estimated that 16% of cases of human infectious intestinal disease (IID) was related to consumption of red meat [Reference Smerdon1] with Campylobacter, Salmonella and Verocytotoxin-producing Escherichia coli (VTEC) O157 reported amongst the most important pathogens of IID [Reference Adak, Long and O'Brien2]. Although chicken products account for more human disease than red meat products, foods of bovine origin have the highest case-fatality rates [Reference Adak3].
In 1995 the Advisory Committee on the Microbiological Safety of Food (ACMSF) [4] advised that all samples of human diarrhoea were tested for E. coli O157. The following year an outbreak in Scotland resulted in 512 cases, of which 22 died, 17 as a result of the outbreak [Reference Cowden5]. The Pennington Report [Reference Pennington6] was commissioned, which reported on the outbreak, advised on the implications for food safety and highlighted the need for livestock prevalence data.
Risk assessment is the basis for Codex risk management decisions and estimates of the prevalence of carriage of foodborne pathogens are required to determine the prevalence of contaminated animals entering the food chain [7]. The abattoir is one of the primary steps in the ‘farm-to-fork’ process and surveillance of pathogen entry will allow more effective implementation of control strategies by risk managers. Two separate red-meat abattoir surveys were commissioned by MAFF (Ministry of Agriculture Fisheries and Food) to investigate foodborne pathogens in ruminants and pigs during 1999–2000 [Reference Davies8, Reference Paiba9] Following this a single study was undertaken in 2003, to provide estimates of the prevalence of carriage of VTEC O157, Salmonella, Campylobacter, Yersinia enterocolitica in cattle, sheep and pigs. The findings from the 2003 study will be discussed in this paper.
MATERIALS AND METHODS
Sample size
Sample-size calculations were performed to estimate the number of samples to collect. The number of samples was determined to enable detection of a difference in prevalence from the figures produced by the 1999–2000 abattoir surveys for the various organisms [Reference Paiba and Gibbens10, Reference Dalziel11]. Because Salmonella in cattle and sheep and VTEC O157 in pigs were isolated at low prevalence levels of <1%, it was not possible to produce feasible figures to monitor a change in prevalence. Therefore, sample-size estimates were undertaken to detect a 1% prevalence ±1% or 0·5% with 95% confidence. Sample sizes were increased by 6% and 0·5% for cattle and sheep respectively to take into account empty rectal samples [Reference Paiba and Gibbens10]. The total sample size was 7616 – comprising 2736 cattle, 2820 sheep and 2060 pig samples.
Abattoir recruitment and schedule of sampling
All 327 eligible red-meat abattoirs abattoirs in Great Britain that slaughtered cattle, sheep and pigs were contacted. Abattoirs were excluded which slaughtered cattle aged >30 months [Over Thirty Month Scheme (OTMS)] as these animals would not enter the food chain. The number of samples collected from each abattoir was proportional to its throughput and if less than two samples were calculated for the 12-month study period, the abattoir was excluded. Although 144 abattoirs agreed to participate, a further 51 were excluded due to low throughput.
Participation bias was assessed using a questionnaire at the time of recruitment and from known data.
The sampling schedule was reviewed and appropriately revised to take into account non-submission of samples and abattoir closures. Where an abattoir ceased trading during the period of the study, its allocation of samples was rescheduled to other participating plants. No additional abattoirs were recruited.
A maximum of four samples was collected on any one occasion to prevent clustering of samples from the same herds and flocks. In addition, samplers were instructed to randomly select animals throughout the course of the day. They were also instructed that no more than one sample should be obtained from any one farm of origin on the same day. If very few animals were presented for slaughter then systematic sampling was undertaken from each quarter of the day's kill.
Sample and data collection
A standard protocol for collecting samples of rectal contents from both cattle and sheep and caecal samples from pigs was developed based on the previous abattoir surveys [Reference Davies8, Reference Paiba9, Reference Dalziel11]. For cattle and sheep, faecal material was milked into the distal rectum. A short section was tied using cable ties above and below the faecal ball and the proximal end was severed from its connection with the large intestine. The entire section including the anus was collected. For pigs, the caecum was located and intestinal contents milked into the closed end of the caecum. This was tied-off using cables and the sealed caecum detached from the remaining intestine. Each sample was double-bagged and labelled with a unique barcode.
A sample collection form, labelled with the same barcodes as the samples was provided for each collection of four animals. The individual animal was identified in the lairage and followed through to the point of sample collection, which allowed correlation of the sample with the retrieved information.
Bacteriology
E. coli O157
Examination for VTEC O157 was carried out as for the previous abattoir survey [Reference Paiba9] and is described in detail elsewhere [Reference Milnes12]. Briefly, 1/10 dilutions of rectal contents in buffered peptone water (BPW) were pre-enriched for 6 h at 37°C followed by immunomagnetic separation using anti-O157 magnetic polystyrene beads (Dynabeads® anti-E. coli O157, Invitrogen, Paisley, UK). Representative non-sorbitol fermenting colonies grown after 18 h incubation at 37°C on cefixime tellurite (Selectavial SV48 and SV49, Mast Diagnostics, Bootle, UK) sorbitol MacConkey agar (CM0813, Oxoid, Basingstoke, UK) were confirmed and typed by somatic antigen serotyping, verocell assay for verocytotoxin production, PCR for vtx1, vtx2 and eae genes and phage typing. Isolates were classed as VTEC O157 if they were confirmed serologically as having the O157 somatic antigen, contained one or both vtx genes, produced verocytotoxin and contained the eae gene.
Salmonella
Salmonella examination was performed according to the method of Davies & Wray [Reference Davies and Wray13], which had been used in the previous abattoir survey [Reference Davies8]. Using 1 g of ruminant rectal contents and 10 g of pig caecal content, 1/10 dilutions in BPW were pre-enriched for 18–20 h at 37°C. Supernatant was inoculated into Diassalm agar plates, incubated at 41·5°C for 24–48 h followed by subculture at 24 h and 48 h onto Rambach agar, which was incubated for 24 h at 37°C. Presumptive Salmonella colonies were confirmed using standard biochemical and serological procedures. Isolates were submitted to VLA Weybridge where serotyping was carried out according to the Kauffmann–White scheme [Reference Popoff and Le Minor14] with phage-typing complying with the Health Protection Agency (HPA), Colindale schemes [Reference Anderson15].
Thermophilic Campylobacter spp
A pea-sized amount of the sample was inoculated into 10 ml Campylobacter enrichment broth [Reference Humphrey, Mason and Martin16] (Exeter; Oxoid, Basingstoke, UK) and incubated at 37°C in a microaerobic atmosphere for 48 h. The broth was then subcultured onto Skirrow's agar plates and incubated microaerobically at 37°C for 48 h. This protocol was a modification of that used for cattle and sheep in the previous survey where enrichment was performed using Preston broth. The method for pigs was also modified from the use of mCCDA agar for isolation. Suspect colonies were examined by Gram stain for typical morphology and for biochemical characteristics (catalase and oxidase activity) and for growth at 25°C and 42°C. Presumptive thermophilic campylobacters (positive for growth at 42°C) were confirmed and speciated as C. jejuni, C. coli, C. lari or C. hyointestinalis using standard VLA tests [17].
Yersinia enterocolitica
Yersinia culture was undertaken using the same protocol as in the previous survey [Reference Paiba and Gibbens10] following the methodology devised by Schiemann [Reference Schiemann18]. In pigs, 1/10 dilutions of 2 g caecal contents in PBS were made and refrigerated at 2–8°C for 14–16 days followed by subculture onto selective CIN (cephsulodin–irgasin–novobiocin) agar and 24 h incubation at 30°C.
However, deviations in sample size and incubation temperature were made to reflect the 1999–2000 survey protocol for ruminant samples, which resulted in 0·5 g in 2 ml PBS with incubation at 25°C for up to 7 days. For all species presumptive colonies were subcultured on to sheep blood agar (SBA) and MacConkey agar and incubated at 37°C for 18–24 h. Identification of Y. enterocolitica was confirmed by colony morphology, urease test and API 20E biochemical strips. All positive isolates were serotyped by the Laboratory of Enteric Pathogens, HPA Colindale.
Analysis
Data were checked for inconsistencies and implausible values. Initial frequencies were tabulated in Microsoft Access and Microsoft Excel with 95% confidence intervals, χ2 and Fisher's exact tests calculated in Stata 8 (StataCorp, College Station, TX, USA).
Participation bias was examined by comparing abattoirs that agreed to participate with those that refused or were unresponsive using χ2 tests or Fisher's exact test on categorical data and Wilcoxon's rank-sum test for continuous data. The variables examined included species slaughtered, abattoir throughput, regional distribution for all species and for individual species, operational status in 1999 and participation in the 1999 survey.
The clustering effect of sampling from batches was examined where all samples from a batch had been tested: VTEC O157, Salmonella (cattle and sheep) and Y. enterocolitica (pigs) using the svymean command in Stata 8 with the population sampling unit set as the sampling visit.
Prevalences were compared between survey periods using χ2 and Fisher's exact tests. If all samples from a batch had been examined in both surveys, the svymean and lincom commands in Stata 8 (with the population sampling unit set as the sampling visit) were used to allow for differing clustering effects in each survey.
RESULTS
Participation
From the 327 eligible plants, 93 (28%) abattoirs participated in the study. Single species abattoirs were significantly more likely to agree to participate than multi-species abattoirs. No significant regional effect on participation response was detected overall. However, when abattoirs were classified by species, regional differences could be detected for pigs (χ2=9·5, P=0·049) with South West abattoirs least likely (33%) and abattoirs in the East most likely to agree to participate (58%).
For all three species, abattoirs agreeing to participate had a significantly higher throughput than those refusing (P<0·05). Abattoirs that participated in the 1999–2000 studies were more likely to agree to participate than those which did not.
Sampling
During the 12-month period of the study, 7703 samples were collected of which 7493 (97%) contained intestinal material.
Prevalence and effect of clustering
The results from the study are displayed in Table 1 which also shows the figures generated by the two studies undertaken in ruminants and pigs in 1999–2000.
Table 1. Showing prevalence of foodborne zoonotic pathogens for 2003 abattoir study with comparable figures for 1999/2000 studies
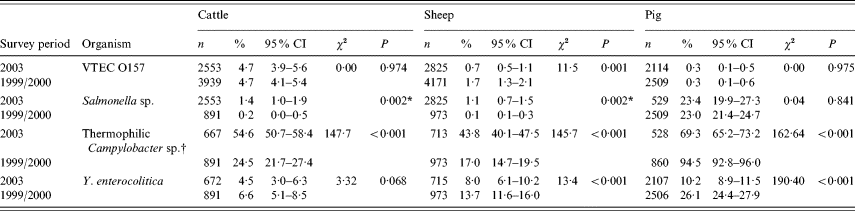
n, Number test; CI, confidence interval.
* Fisher's exact test (two-tailed P value).
† Difference in laboratory methodology between the surveys for ruminants.
For completeness, where more than one sample was tested from the batch of four collected, clustering was accounted for with adjusted and unadjusted results, which revealed that clustering did not affect the results (data not shown).
VTEC O157
Prevalence
The 12-month study produced a total of 148 isolates of VTEC O157 of which 121 were from cattle, 21 from sheep and six from pigs. The prevalence was 4·7% (95% CI 3·9–5·6) for cattle, 0·7% (95% CI 0·5–1·1) for sheep and 0·3% (95% CI 0·1–0·5) for pigs (Table 1). There was a significant difference in prevalence between species (χ2=154, P<0·01). Comparison with results from the previous abattoir surveys revealed no change in carriage for cattle and pigs but a significant decrease (P<0·01) for sheep from 1·7% in the previous survey.
No significant variation in regional prevalence was shown for cattle although prevalence varied from 3·0% in the South West to 6·2% in North East. Numbers were too small to draw conclusions for sheep and pigs.
Seasonality
A significant seasonal effect was detected in cattle where the prevalence varied from 6·4% in summer (June–August) to 3·2% in autumn (September–November) but not in sheep or pigs. Within each season, VTEC O157 isolation was significantly greater in cattle than sheep.
Characterization
The majority of the VTEC O157 isolates, 118/121 from cattle, and all VTEC O157 isolates from sheep and pigs contained the vtx2 gene. Three cattle isolates contained vtx1 without the presence of vtx2. In total, 22 cattle and one sheep isolates contained both vtx1 and vtx2.
The phage types of the VTEC O157 isolates are given in Table 2. For cattle there were three predominant phage types (PT): 21/28, 8 and 34, which accounted for 65% (78/120) of typed isolates. In sheep the number of isolates was smaller with PT4 the most common. Four phage types accounted for the six pig isolates with PT2 recovered from two samples. In the previous surveys, the predominant phage types for cattle in descending order were 21/28, 2, 8, 32, 4, 34; for sheep 4, 32, 21/28, 14 and for pigs 4, 14, 32.
Table 2. Phage types of VTEC O157 isolates by animal species
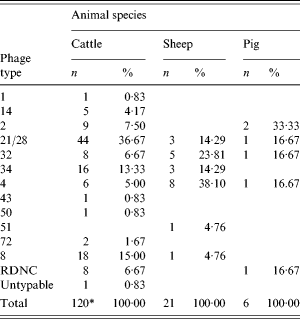
* Excludes one isolate not examined.
Salmonella
Prevalence
The prevalence of carriage of Salmonella was 1·4% (95% CI 1·0–1·9) in cattle, 1·1% (95% CI 0·7–1·5) in sheep and 23·4% (95% CI 19·9–27·3) in pigs – a statistically significant difference between the three species. A significant increase from the previous abattoir surveys was shown for ruminants but not pigs (Table 1).
The 1999–2000 survey reported figures of 0·2% (95% CI 0–0·5) for cattle, 0·1% (95% CI 0·1–0·3) for sheep and 23% (95% CI 21·4–24·7) for pigs. For cattle, the prevalence varied by season from 0·8% in winter to 2·4% in the summer months. In sheep, the spring months saw the highest numbers of isolates. In pigs seasonal prevalence varied from 20·0% in spring to 25·2% in autumn. These changes were not statistically significant.
For cattle, the prevalence of faecal carriage varied from 0·3% in the North West to 3·1% in the South West with a significant regional variation reported (χ2=21·98, P<0·001). In sheep, significant regional variation was also found (χ2=27·54, P<0·001) involving separate regions from cattle. Prevalence ranged from 0·2% in Scotland to 5·6% in the North East. In pigs, no significant regional variation was demonstrated.
Serotypes
There were 36 confirmed Salmonella isolates from cattle, 30 from sheep and 124 from pigs. The most common serovars from cattle were S. Typhimurium, S. Mbandaka and S. Dublin (Table 3). Isolates of S. Typhimurium featured prominently in the spring and summer months. From sheep the most common serovar was S. enterica subsp. diarizonae 61:k:1,5,7 (and associated incomplete antigenic structures) and S. Dublin. In pigs, S. Typhimurium and S. Derby were the most common serovars accounting for 79% of isolates. Within S. Typhimurium, in pigs serovars DT104 and 193 were commonly isolated representing, respectively, 9·7% and 14·5% of isolations.
Table 3. Salmonella serotypes in cattle, sheep and pigs
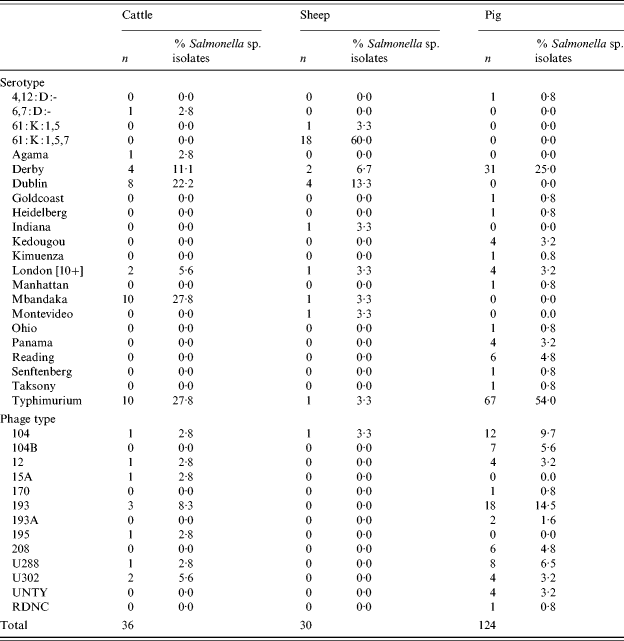
There were no isolations of Salmonella Enteritidis, Hadar, Infantis or Virchow from any species. These serovars together with Typhimurium are those most commonly isolated from human cases of salmonellosis in the European Union and are considered to be serovars of high public health significance. Using this definition, the prevalence of Salmonella serovars of high public health significance in large animal species in Great Britain was 0·4% (95% CI 0·2–0·7) in cattle, 0·04% (95% CI 0·0009–0·2) in sheep and 12·7% (95% CI 10·0–15·8) in pigs.
Thermophilic Campylobacter spp
The prevalence of thermophilic Campylobacter spp. (C. jejuni, C. coli and C. hyointestinalis) was 54·6% (95% CI 50·7–58·4) in cattle, 43·8% (95% CI 40·1–47·5) in sheep and 69·3% (95% CI 65·2–73·2) in pigs (Table 1). No C. lari was found. The species effect was highly significant (χ2=79·9, P<0·001).
Comparing these with the previous survey results revealed a significant increase in ruminant faecal carriage and a significant decrease in porcine carriage. However, it should be noted that changes were made to the methodologies for Campylobacter isolation between surveys.
There were no significant differences in seasonal prevalence for cattle and pigs. The maximum prevalences were 57·4% for cattle in summer and 74·7% for pigs in autumn. Carriage in sheep varied from 39% in summer to 52·8% in winter, a variation that was statistically significant (P=0·046).
In cattle and sheep, C. jejuni was the predominant species isolated representing 81% of all cattle and 65% of all sheep Campylobacter isolates. The remainder were primarily C. coli. In cattle 7% of isolates were C. hyointestinalis but none were observed in sheep.
In pigs, 90% of Campylobacter isolates were C. coli (Table 4), the remainder were mostly C. jejuni or C. hyointestinalis. This is consistent with the previous survey for pigs.
Table 4. Thermophilic Campylobacter isolates by species
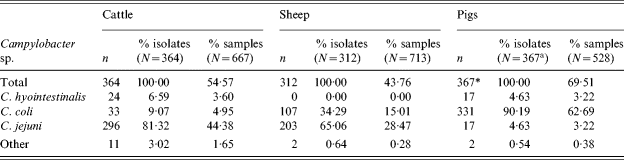
* Campylobacter sp. isolated from 366 samples, one sample was positive for two species.
Y. enterocolitica
In cattle there were 30 isolates of Y. enterocolitica representing 4·5% (95% CI 3·0–6·3) of the submissions (Table 1). This is a statistically non-significant reduction from the previous abattoir survey that reported a prevalence of 6·6% (95% CI 5·1–8·5). In sheep, the 2003 prevalence was higher, 8·0% (95% CI 6·1–10.) which represents a significant reduction from the previous survey prevalence of 13·7% (95% CI 11·6–16·0) (χ2=13·39, P<0·001). In pigs, the 2003 prevalence of 10·2% (95% CI 8·9–11·5) is significantly lower than the 26·1% (95% CI 24·4–27·9) reported by the previous survey (χ2=190, P<0·01). Examination of the seasonal pattern of excretion (Fig. 1), revealed a significant difference between the seasons in cattle and pigs. In general, carriage peaked during the colder winter months with troughs occurring during the summer months for sheep and pigs. For cattle, the autumn months appeared to provide the lowest carriage.
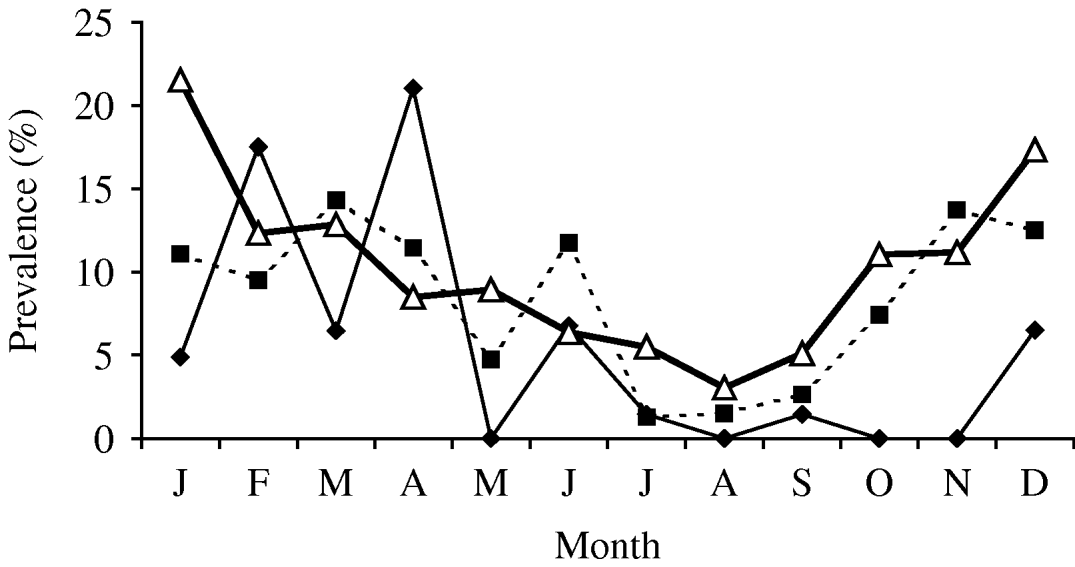
Fig. 1. Prevalence of Yersinia enterocolitica by month. –♦–, Cattle; –■–, sheep; –△–, pig.
Y. enterocolitica biotyping
Biotyping of Y. enterocolitica isolates was undertaken for all host species. This data is comparable with the biotypes of isolates from the previous survey [Reference McNally19]. Y. enterocolitica biotype (BT) 1a was the most frequently isolated biotype from livestock in both surveys.
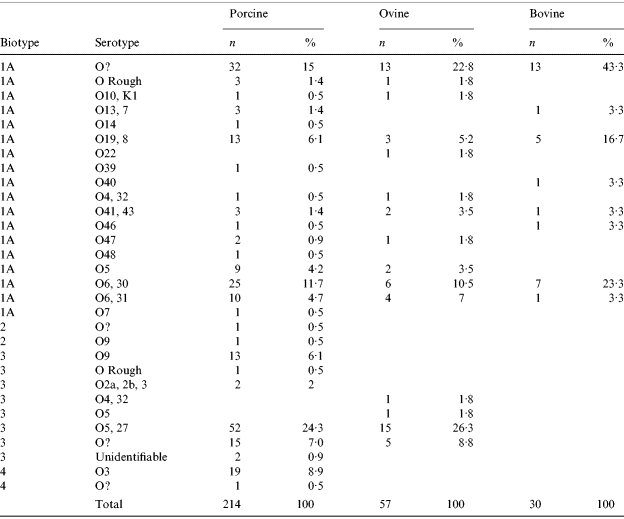
In cattle, all isolates were BT1a. In sheep, 61% of isolates were BT1a with the remaining 39% BT3 mostly serotype 05,27. A higher number of Y. enterocolitica isolates was reported in pigs than in ruminants with 214 isolates recovered. The most common BT was 1a accounting for 50% (107) of the porcine isolates. The putative-pathogenic BT2, 3 and 4 strains accounted for 1%, 40% and 9% respectively of the remaining isolates. The most common (24%) strain type in pigs was BT3 (serotype 0:5,27) (Table 5).
Table 5. Yersinia enterocolitica serotypes for cattle, sheep and pigs
DISCUSSION
All abattoirs slaughtering cattle, sheep and pigs were invited to participate in the study with 93 enrolled from 327 eligible abattoirs. However, the participating abattoirs were estimated to represent a large proportion of the slaughter population (63% of cattle, 58% of sheep and 86% of pigs) and therefore the target population, i.e. animals entering the food chain in Great Britain. The median age of cattle was 24 months; pigs 6 months, and with sheep 71% of samples came from lambs born within the season. No further details were given as the purpose of the study was to estimate the carriage of foodborne pathogens in the animals presented for slaughter for human consumption. The participation rate reflected the voluntary nature of the study and was similar to that of the previous study.
Four samples were collected at each visit and for Salmonella in ruminants, Y. enterocolitica in pigs and VTEC O157 all samples were examined. The sample collection protocol was devised to reduce the effect of clustering. During the analysis the potential effect of clustering was investigated with little effect noted.
The prevalence of VTEC O157 in both cattle and pigs was unchanged from the previous VLA abattoir studies with a significant reduction noted for sheep. However, the number of isolates from both studies was relatively small. The study design of the 2003 abattoir survey was based on the previous study to allow valid comparisons to be made. In addition, the same laboratory methodology was used in both. It would therefore seem unlikely that the change in prevalence in sheep samples was associated with any changes in methodology.
Comparisons with other studies should be made with caution due to differences in study design and in bacteriological methods applied. However, previous abattoir studies looking at faecal samples have provided various figures with 7·5% reported by a Canadian study [Reference Van Donkergoed, Graham and Gannon20], 9·2% by a Scottish study [Reference Ogden, MacRae and Strachan21] and Italian studies varying from 3·6% to 16·6% [Reference Conedera22, Reference Bonardi23]. In general, detectable carrier levels in individual cattle are low, which may be due to the transient nature of shedding [Reference Clifton-Hadley24–7]. Therefore, large numbers of samples are required to produce valid results.
The most common phage type identified in cattle by this survey was 21/28, which represented 37% (44/120) of typed isolates. In the previous abattoir survey, both PT21/28 and PT2 were recovered in equal numbers with each representing 18% of isolates. In the 2003 survey, reduced numbers of PT2 were seen, a trend mirrored by human cases. Previously, human isolates of VTEC O157 have belonged to a relatively select number of phage types with PT2 and PT49 more commonly seen [Reference Willshaw28]. However, between 1995 and 2003 PT2 has declined in England and Wales from being the most prevalent phage type isolated from humans in 1995 to being the third most prevalent after PT21/28 and PT8 in 2003 followed by PT32 and PT4. In Scotland, a similar pattern has been seen with PT21/28 becoming predominant over PT2 and PT8 by 2003 [Reference Smith29]. Recording the phage types carried by foodborne animals may allow early warning of changing patterns of human disease.
Of the three species involved in the study, VTEC O157 carriage was greatest in cattle. This supports findings of previous studies [Reference Davies and Wray13] and the theory that cattle are the predominant reservoir of this organism [Reference Hancock26, Reference Chapman30, Reference Heuvelink31].
Seasonal variation was seen in both cattle and sheep with VTEC O157 carriage highest during the summer period. This agrees with the finding of the previous abattoir survey and with several other researchers who indicate that prevalence is greatest during the summer months [Reference Schouten25–Reference Mechie, Chapman and Siddons27, Reference Vali32, Reference Barkocy-Gallagher33]. This mirrors the pattern noted in human cases in the United Kingdom [Reference Willshaw34]. Although the months of June and July were associated with peak faecal carriage in cattle, a smaller rise was noted in November and December producing a bimodal distribution for cattle, similar to the findings of a study of Dutch dairy farms [Reference Schouten35]. The late autumnal/winter rise was also noted by Scottish researchers [Reference Synge36]. In fact, various studies on Scottish cattle have shown that prevalence is greatest in winter. Ogden et al. [Reference Ogden, MacRae and Strachan21] suggested that the winter rise may be associated with housing and close contact between the animals. Cattle, within our study, were sourced from Great Britain including Scotland and husbandry systems are thought to be similar across the five regions involved. However, with colder winters occurring in Scotland, cattle are housed for longer than their southern counterparts and this may allow for the winter rise seen in the Scottish studies.
Despite an apparently low prevalence of ruminant Salmonella reported by the study, this represents a significant rise from the findings of the 1999–2000 survey. There was no change in test methodology or testing laboratory between surveys. The rise in carriage in ruminants since the last survey may be associated with an increase in livestock movement involved in restocking following the outbreak of foot-and-mouth disease in 2001, which could have allowed spread of colonized animals.
In pigs, carriage was significantly greater than in ruminants. Abattoir surveys from other countries also report higher prevalence in pigs than in ruminants. Due to differences in study design and culture techniques it is often difficult to compare the findings with other national studies. In general, the relatively high prevalence found in slaughter pigs in the United Kingdom does not coincide with similar findings in other countries [Reference Davies8]. However, Canadian studies have reported carriage varying from 5% to 80% [Reference Letellier, Messier and Quessy37, Reference Mafu38] with a recent Italian study reporting carriage of 36% from 150 caecal samples [Reference Bonardi39]. A survey of Danish pigs in 1998–1999, after the implementation of a control policy described carriage of 3·4% in caecal samples [Reference Christensen40] with a German study reporting 3·7% carriage from rectal swabs [Reference Kaesbohrer41].
In total, 36 Salmonella isolations were made from cattle, compared with just two in the previous survey. The predominant serovars in cattle were S. Mbandaka, which is usually feed related and asymptomatic, S. Typhimurium and S. Dublin. A rise in S. Dublin has been reported recently in cattle and this serotype tends to increase in prominence in cycles between peaks in other ‘epidemic’ serotypes but there had been no increase in total annual reports of Salmonella in cattle in the period between surveys. In sheep, there were 30 isolates including 19 isolates (63%) of S. enterica subsp. diarizonae. This serovar was the most frequent noted by the Salmonella in Livestock Production in GB Report for 2003 and has been reported in cases of clinical disease and from healthy animals [42]. S. Dublin was also noted, perhaps reflecting the situation in cattle. In pigs the predominant serotypes were S. Typhimurium (54% of isolations) and S. Derby (25%), the former a notable zoonotic serotype whilst S. Derby is an unusual cause of human disease, mirroring the findings of the previous survey.
Seasonal influences were noted in Salmonella carriage, with summer the period of peak excretion in cattle. This may reflect increased exposure to the organism at pasture, e.g. through slurry spreading on grazing ground. In sheep, the spring period had the highest percentage of isolates which may be related to increased susceptibility at lambing and consequent spread to slaughter animals.
The prevalence of thermophilic Campylobacter spp. reported by this survey was 54·6% in cattle, 43·8% in sheep and 69·3% in pigs and in all species represents a significant change from the 1999–2000 abattoir survey. In the previous study, the faecal carriage reported for cattle and sheep was respectively 24·5% and 17·0% suggesting a significant increase in Campylobacter carriage. However, the methodology employed for ruminant samples in the previous survey was thought to have underestimated the true prevalence. Campylobacters are notoriously sensitive to any environmental stresses [Reference Griffiths and Park43] making isolation difficult particularly when present in low numbers and in material like faeces, heavily contaminated with other competitive flora. Comparable results were produced by Stanley and others [Reference Stanley48] from an abattoir survey in 1998 using the same selective broth as the 1999–2000 survey, 26·7%. By including an enrichment step, isolations increased to 89·4% of samples.
A Campylobacter Isolation Validation Study indicated that if the 2003 methodology had been used during the 1999–2000 survey on ruminant samples then the probable isolation rates for cattle and sheep would have been 68% and 56% respectively which would be consistent with the results obtained in 2003 [Reference Paiba44].
Despite these issues, in this survey for the first time, all three species were examined using the same technique in the same laboratory, allowing comparability of results between species. Therefore, figures produced by this survey allow baseline prevalence estimates for carriage of thermophilic Campylobacter spp. in cattle, sheep and pigs at slaughter in Great Britain and indicate that in all three hosts campylobacters of public health significance are common components of the gut flora. The study identified C. jejuni as the predominant isolate from ruminant samples with C. coli predominant in pigs. About 90% of human cases of campylobacteriosis are due to C. jejuni with 10% due to C. coli. A significant seasonal difference was reported in sheep with Campylobacter carriage highest during the winter period. In addition, differences in seasonal trends in cattle were noted, albeit they were not statistically significant; the relevance of this is unknown. In human infections a spring or summer peak is well recognized [Reference Nylen45, Reference Miller46] which in the United Kingdom is seen 6–8 weeks before the August Salmonella peak [Reference Adak3]. A study of four dairy farms in Lancashire reported spring and autumnal peaks [Reference Stanley and Jones47], but no seasonality was found in a study of animals reared for beef [Reference Stanley48], reflecting the findings of this study where 91% were beef breeds.
Y. enterocolitica was isolated significantly more frequently from sheep than from cattle, a finding also reported by Paiba & Gibbens [Reference Paiba and Gibbens10]. However, in this study there was a significant decrease in the prevalence of Y. enterocolitica in sheep and pigs compared to the previous studies in 1999–2000.
Y. enterocolitica is a ubiquitous organism with only a few bioserotypes associated with human disease [Reference Bottone49]. Although a known foodborne pathogen, infection is associated with consumption of pork products rather than lamb or beef as pigs are the major animal reservoir of pathogenic strains [Reference Letellier, Messier and Quessy37, Reference Funk50]. The reduced prevalence in pigs is therefore more notable in terms of zoonotic implications than the change noted in sheep. Comparisons between the two studies may suggest that prevalence is not static and that fluctuations do occur within food- producing animals.
The most commonly identified biotype was 1a which compares with the results from the previous abattoir survey. Although traditionally thought of as non-pathogenic, there is growing opinion which sheds doubt on this view and in the period 1999–2000 this was the predominant biotype isolated from human cases [Reference McNally19]. BT1a was the only biotype isolated from cattle during the 2003 study, and was the predominant cattle biotype in the 1999–2000 study. However, in the previous study BT3 O:5,27 was also isolated.
In previous UK studies, BT3 (serotype O:9 and O:5,27), BT2 (serotype O:9) and BT4 (serotype O:3) have been the predominant strains associated with human disease [Reference Prentice, Cope and Swann51]. These strains were identified in 40% (85) of pig Y. entercolitica isolates and 26% (15) of sheep isolates. Only BT1a was recovered from cattle. BT3 (O:5,27) was the most common putatively pathogenic biotype carried by surveyed pigs and sheep, concurring with 1999–2000 abattoir survey results. However, HPA data from the period 1999–2000 indicated that in human cases, 53% were BT1a, 24% BT3 (O:9) and 19% BT4 (O:3) with only 1·2% BT3 (O:5,27) [Reference McNally19] suggesting that the zoonotic sources of yersinia infection in humans require further investigation.
This study follows on from two abattoir surveys undertaken by the VLA in 1999 and 2000. For the 2003 survey, changes to the laboratory methodologies were made where appropriate, but in general, the techniques employed were similar. The figures generated by the first surveys were used when calculating the required samples sizes allowing fewer samples to be collected while employing epidemiological principles to ensure the validity of the study. The VLA laboratories are UKAS accredited indicating that standards are high with quality assurance systems in place. Comparisons between the two study periods can therefore be made. This study has produced estimates of the prevalence of carriage of four foodborne pathogens in slaughter animals in Great Britain. The study design used can be replicated and used as a template for future abattoir surveillance. With the use of the same laboratory protocols the results would be comparable and allow monitoring of foodborne pathogens at the level of the abattoir. Sampling at the level of the abattoir gives an indication of the entry level of pathogens to the human food chain and this information can be used in risk assessment for foodborne disease with the figures used to assess the effectiveness of risk management strategies.
This study indicates that there has been no change in intestinal carriage of VTEC O157 in cattle and pigs in the 5 years since comparable abattoir studies were undertaken. In sheep, carriage has decreased. Cattle remain the species with highest intestinal carriage of this organism. For Salmonella and thermophilic Campylobacter, a significantly greater proportion of pigs carry these pathogens than ruminants at slaughter. The prevalence of Y. entercolitica has decreased significantly in pigs and sheep. For all organisms, the samples were taken from healthy animals intended for human consumption and the results presented will be representative of this target population. However, prevalence estimates within other groups cannot be extrapolated from this study.
ACKNOWLEDGEMENTS
The authors thank the abattoirs and their staff for participating in this study. We gratefully acknowledge the staff of the Meat and Livestock Commission for collecting the samples and data, with particular reference to Ron Milne for coordinating this work. The VLA Regional Laboratory staff are gratefully acknowledged for their assistance in both sample and data collection and the laboratory examination of samples. Kathy Speed and Katherine Sprigings are thanked for their assistance. We are also grateful to the staff of the Health Protection Agency Colindale for phage-typing of VTEC O157 and serotyping of Yersinia isolates. This project was funded by the Department for Environment, Food and Rural Affairs (Project FZ2009).
DECLARATION OF INTEREST
None.








