INTRODUCTION
Montenegro, formerly a state of Yugoslavia, is a country in South-eastern Europe and has an estimated population of 640 000. Podgorica is the capital and largest city of Montenegro with 136 000 inhabitants and is the administrative centre of Montenegro as well as its economic and cultural focus. In 2006, Montenegro was declared an independent state and, in 2007, became the 47th member state of the Council of Europe [1]. The Government of Montenegro has prioritized the development of the country for elite tourism. Like any tourist destination, Montenegro is concerned about its reputation as a safe destination in terms of visitors' health.
Despite wealthy economies and access to proven drinking water-treatment technologies, significant outbreaks of waterborne intestinal disease still occur in the occidental world [Reference Mackenzie2–Reference Pollock4]. Waterborne disease outbreaks often involve acute gastroenteritis [Reference Beaudeau5]. The symptoms of acute gastroenteritis range from mild, watery diarrhoea to severe febrile illness characterized by vomiting, diarrhoea and prostration. Viral infection is the most common cause of epidemic gastroenteritis worldwide and many outbreaks are due to norovirus [Reference Atmar and Estes6] although children are notably susceptible to rotavirus infection [Reference Giaquinto7, Reference Anderson8].
On 24 August 2008, the Institute of Public Health (IPH) of Montenegro was informed of an unusually high number of patients presenting with acute gastroenteritis, at both the Infectious Disease and Paediatric hospitals in the city of Podgorica. The IPH promptly initiated an outbreak investigation and informed the World Health Organisation (WHO) Country Office. The WHO Regional Office for Europe, in consultation with Montenegrin authorities and with the support of collaborating national public health institutes in the region, contributed to the outbreak investigation through the deployment of an international multi-disciplinary team from 7 to 19 September 2008. We conducted epidemiological, environmental and microbiological investigations to determine the source and extent of the outbreak and to guide appropriate short- and long-term control and preventive measures.
METHODS
Descriptive epidemiological investigation
Physicians in Montenegro are required to report infectious diseases and syndromes both to the regional public health office and to IPH in Podgorica. Acute gastroenteritis is subject to mandatory notification by health centres/doctors and is broadly defined as ‘anyone presenting to their health centre/doctor with the following symptoms: abdominal pain, vomiting and/or nausea, diarrhoea and/or fever’.
Descriptive epidemiology was performed on reported cases of acute gastroenteritis with dates of onset from 23 August to 7 September (outbreak period). Furthermore, initial interviews were conducted with a subset of these cases, available family members and the nearest available neighbours. The purposes of the interviews were to obtain information on symptoms and possible exposures, to learn about the extent of the outbreak and, if possible, to establish a source of infection. The explosive and widespread occurrence of cases and results of the initial interviews in several of the early cases reported to IPH, led to the hypothesis that municipal tap water was the probable source of infection. Non-infectious aetiology was excluded.
Analytical epidemiological investigation
An age- and neighbourhood-matched case-control study was performed on 18–24 September to test the hypothesis that the source of the outbreak was municipal drinking water.
A case was defined as ‘acute gastroenteritis with a date of onset from 23 to 25 August (outbreak peak) who had the earliest date of onset in their household and who lived in North-western districts of Podgorica’. The study was confined to North-western districts of Podgorica because these were almost exclusively served by a water source (Mareza), which initial assessment deemed to be a potential source of contamination of the municipal water supply. Microbiological testing of the municipal drinking water indicated positive results for coliforms at points in the distribution system, which was served almost exclusively from the Mareza water source. Furthermore, cases resided in all districts of the city and this water source was the only one that could provide water for all areas of the city.
Controls were identified in neighbours of cases. Regarding logistic feasibility and study power considerations, three controls were selected for each case according to their age group (0–20, 21–40, ⩾41 years). Individual matching on neighbourhood was performed for practical reasons and to control for socioeconomic status, and on age to increase study efficiency as the outbreak disproportionately affected younger persons. Neighbours also served as a non-probability sample in order to obtain a crude estimate of the size of the outbreak.
Information on drinking water habits (e.g. consumption of unboiled water at home or at work and consumption of bottled water) was collected from cases and controls by use of a questionnaire. Data were entered into EpiInfo version 6.3 (CDC, USA) and analysed with the statistical software package Stata version 10 (StataCorp, USA). To study the bivariable associations of water-consumption habits and acute gastroenteritis, we employed exact conditional logistic regression, using the exlogistic command in conjunction with the group option, for dichotomous variables, and the Wilcoxon rank-sum test for continuous variables. The latter test was chosen because it required no assumption on the distribution of the explanatory variables. We included neighbours as cases if they reported symptoms of acute gastroenteritis during the outbreak period. It was considered likely that their illness was associated with the outbreak and their inclusion improved power. To assess the influence of different approaches to case ascertainment, a sensitivity analysis was conducted that considered only those cases that were reported to the surveillance system.
Microbiological samples from cases
Faecal samples were obtained from 200 cases that presented to healthcare facilities, with dates of onset of illness from 24 to 26 August 2008, 2–4 days after the onset of symptoms. These samples were microbiologically investigated for pathogenic bacteria including Salmonella, Campylobacter, Shigella or Yersinia spp., at IPH. Faecal samples were assayed using ELISA kits (bioMérieux, France) for both rotavirus and adenovirus at IPH. In addition, 38 faecal samples from a subset of cases presenting during 24–26 August were sent to the Istituto Superiore di Sanità, Rome, Italy for norovirus testing. For all samples, norovirus detection was performed by reverse transcription and polymerase chain reaction (RT–PCR) amplification of both ORF1 and 2. Viral RNA was extracted from stools using QIAamp Viral RNA mini kit (Qiagen, Germany), and subjected to RT–PCR amplification of a 327-bp area fragment in ORF1 (RNA-dependent RNA polymerase region) using degenerate primers JV12-JV13 [Reference Vinje and Koopmans9] and SuperScript One-Step RT–PCR with Platinum Taq (Invitrogen, USA), according to the manufacturers' instructions. To confirm diagnosis and identify possible multiple norovirus genotypes, samples were also examined by a second RT–PCR assay targeting the ORF2 capsid protein region (315-bp fragment) using G1SKF/G1SKR and G2SKF/G2SKR primer sets specific for norovirus genogroups I and II, respectively [Reference Kojima10]. Cycles for ORF1 RT–PCR reactions were as follows: reverse transcription at 45°C for 30 min, followed by denaturation at 94°C for 2 min, and 40 cycles at 94°C for 45 s, 37°C for 45 s, and 72°C for 1 min, followed by a final elongation step of 72°C for 7 min. For ORF2 RT–PCR, cDNA was amplified through 40 cycles at 94°C for 45 s, 50°C for 45 s, and 72°C for 1 min.
Genotype characterization was accomplished by nucleotide sequencing of the amplified ORF2 region and comparison of sequences with both GenBank (www.ncbi.nlm.nih.gov/Genbank/) and the Food-borne Viruses in Europe (FBVE) databases (www.hypocrates.rivm.nl/bnwww/divine-event/index.html). The DNA band was separated and excised from a 2% agarose gel, purified with a High Pure PCR Product Purification kit (Roche, Switzerland), and sequenced using the PCR primers with the BigDye Terminator Cycle Sequencing Ready Reaction kit version 3.1 (Perkin Elmer, Applied Biosystems, USA) in an automated sequencer (ABI Prism 310 DNA sequencer, Applied Biosystems).
Environmental investigation
The municipal water supply was assessed through field visits and interviews with responsible staff. Standard operating procedures for disinfection, distribution and contingency plans were examined. Raw and final drinking-water quality is routinely tested for coliforms and enterococci once every 2 weeks, at 18–19 fixed sampling points. Because the outbreak was thought to be associated with the municipal water supply, the municipal drinking water was hyperchlorinated on 25 August. From this date, daily samples from 8–9 of the 18–19 fixed sampling points of the municipal water supply were taken. Four drinking water samples (5 litres) were collected from the municipal supply of Podgorica on 27 August and 22 September. These samples were sent to the French Food Safety Agency in Paris for molecular analyses (RT–PCR) of adenovirus, rotavirus, norovirus and hepatitis A virus [Reference Perelle11].
RESULTS
Descriptive epidemiological investigation
During the outbreak period (23 August to 7 September), 1699 cases of acute gastroenteritis were reported to IPH (Fig. 1). The number of cases of acute gastroenteritis typically reported to IPH during this season is between 10 and 20 reports per week. The median age of cases was 13 years [inter-quartile range (IQR), 5–26 years]; 816 (48%) were male. Of the reported cases interviewed during initial investigations, and for whom the information was available (n=88), the median duration of symptoms was 26 h (range 7–96 h); 73 cases (83%) reported vomiting, 70 (80%) diarrhoea, and 55 (62%) reported both of these symptoms. Pyrexia was not reported.
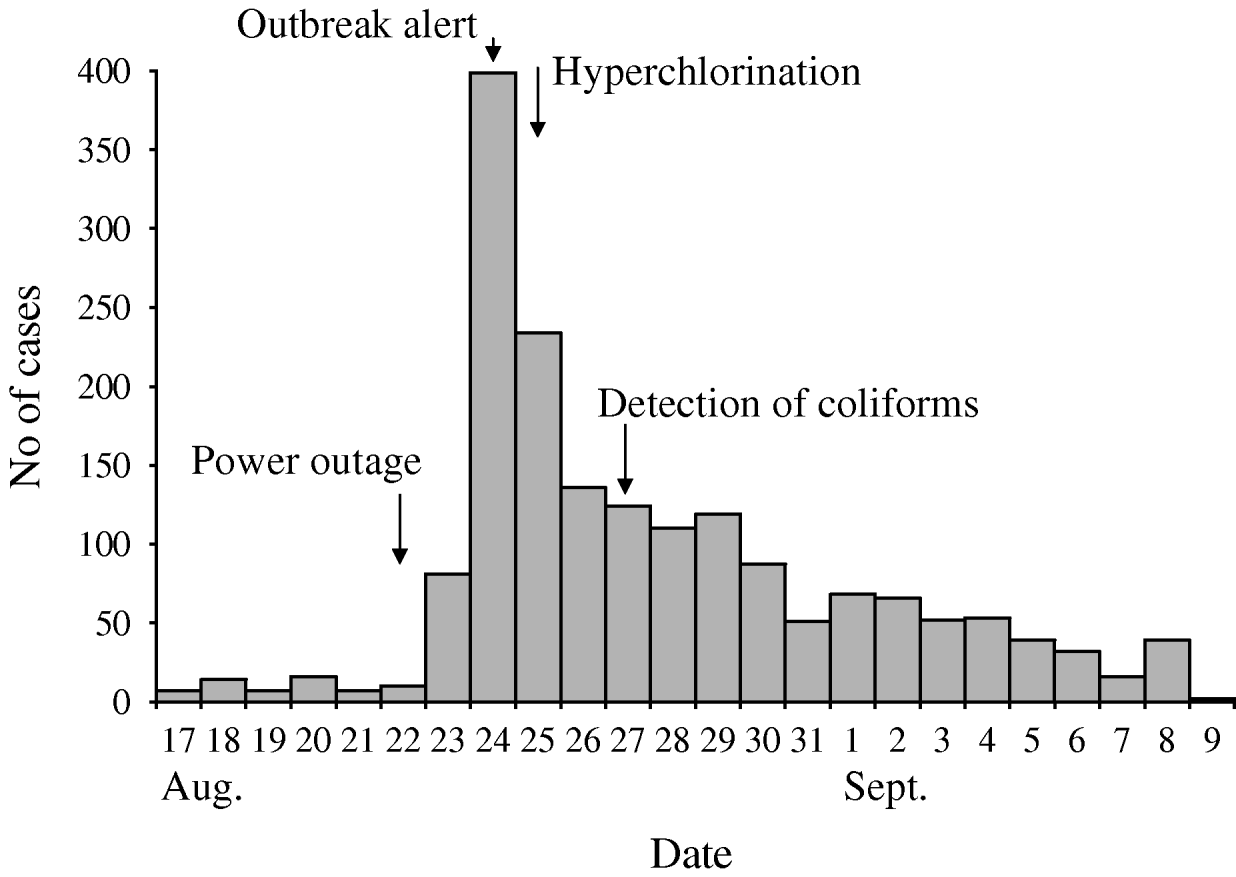
Fig. 1. Date of onset of 1699 ‘acute enterocolitis’ cases notified to the Institute of Public Health, Podgorica, Montenegro, 2008.
Analytical epidemiological investigation
In the matched case-control study, 43 patients reported to IPH with acute gastroenteritis and 129 of their neighbours were interviewed. Of the latter, 40 (31%) had symptoms compatible with acute gastroenteritis during the outbreak period (23 August to 7 September) and were included in the analysis as cases. Median age of the 83 cases was 16 years (IQR 8–27 years); 41 (49%) were male. In the analysis, illness had a positive association with drinking unboiled municipal tap water [matched odds ratio (mOR) 11·2, 95% CI 1·6–∞), (Table 1). Controls more frequently reported drinking bottled water (mOR 0·3, 95% CI 0·1–0·8) than cases (Table 1). Drinking unboiled water from the municipal water supply was almost a universal habit; all 83 (100%) cases and 80 (93%) of the 89 controls reported to have done so during the week before the outbreak. Of the nine controls that denied consumption of unboiled municipal water, eight reported drinking bottled water (one denied any water consumption). Thus, drinking only bottled water was highly protective (mOR 0·02, 95% CI 0·0–0·8, Table 1). Furthermore, a significantly greater number of glasses of bottled water were consumed by controls than by cases (0·9 vs. 0·7 glasses, Table 1). The sensitivity analysis that included only cases reported in the surveillance system yielded estimates exhibiting the same trends as the previously mentioned results, without reaching statistical significance. Extrapolating the proportion of neighbours with symptoms of acute gastroenteritis (31%) to the population of Podgorica (136 000) yielded a crude estimate of up to 42 000 individuals affected by this outbreak.
Table 1. Results of an individually matched case-control study for an outbreak of acute gastroenteritis in Podgorica, Montenegro, 2008
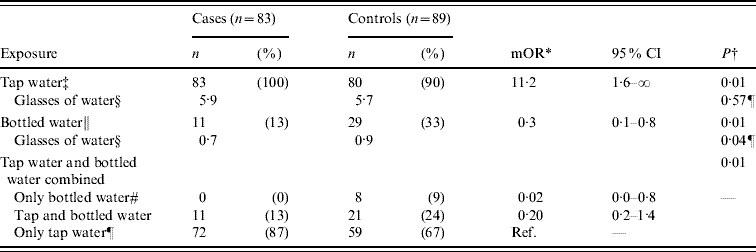
mOR, Matched odds ratio; CI, confidence interval.
* Median unbiased estimate of mOR inexact conditional logistic regression.
† P value of exact conditional logistic regression if not otherwise stated.
‡ Drinking unboiled water from the municipal water supply in the week before the outbreak started (18–24 August).
§ Mean glasses of water per day.
¶ P value of Wilcoxon rank-sum test.
∥ Drinking bottled water in the week before the outbreak started (18–24 August).
# Drinking bottled water, but not unboiled water from the municipal water supply in the week before the outbreak started (18–24 August).
Microbiological samples from patients
Of 200 faecal samples, 16 (8%) had a positive stool antigen result for rotavirus (all children) and two (1%) samples from adults tested positive for adenovirus. None of the samples yielded any positive results for pathogenic bacteria. Norovirus was identified in 23/38 (60%) faecal samples by one or both of the RT–PCR methods described. Norovirus genogroup II strains were recovered from 18/38 (47%) faecal samples. Of these 18, seven strains were typed by sequence analysis and found to belong to genotype GII.2 (three), GII.4-2006b (two) and GII.17 (two). In addition, norovirus genogroup I strains GI.3 (one), GI.2 (three) and GI.13 (one) were found in the stools of 6/38 patients, including one patient also shedding GII norovirus. In several cases, the amount of DNA generated by PCR was not enough to enable sequencing; however, using genogroup-specific ORF2 PCR, it was possible to ascertain a specific correlation with norovirus genogroups I or II. Co-infection with genogroups I and II strains was found in two further patients. In two additional samples, norovirus was detected by ORF1 RT–PCR only. However, in general the ORF1 RT–PCR was less sensitive, yielding a positive result in only 13 cases out of 38 stools examined. Of these, 10 were positive for GII ORF2, and one was positive for GI ORF2 by RT–PCR. The intra-cluster identity of the 10 norovirus sequences assigned to genotypes GII.2 (three sequences), GII.4-2006b (two), GII.17 (two) and GI.2 (three) was between 99% and 100%.
Environmental investigations
There are three sources of drinking water that serve the city of Podgorica. The largest water source (Mareza) produces karstic spring water (1100 litres/s) while the other two sources produce groundwater (340 and 290 litres/s). The distribution systems of all three are connected and complex. Water is chlorinated to 0·4 mg/litre at the pump station of Mareza, although there is no main holding tank for treated water before distribution and there is no measure of free residual chlorine. The rationale for having a holding tank and subsequent measurement of free residual chlorine is to enable sufficient contact time for free chlorine to disinfect drinking water, before it enters the distribution supply to consumers. According to the water supply company, 40% of the water is possibly lost in the water distribution system before reaching the consumers. There was no farming activity in the vicinity of the catchment areas of the three sources but there was a small village 300 m from the largest water source (Mareza) and 50 m higher than this source. According to the IPH, the village had no connection to the main sewage system but each dwelling had porous, septic tank systems.
Tests on municipal drinking water did not yield positive microbiological results before the outbreak (last date tested: 18 August). However, results from 27 August indicated several positive results (3/8) for coliforms in the chlorinated Mareza distribution system. Furthermore, no chlorine could be detected in these water samples, taken at different points in the distribution system, despite the samples being from chlorinated water. Furthermore, electrical pump failures at two of the water treatment works at Mareza were reported by the Department of Sanitation on 22 and 27 August.
The molecular screening of the four water samples collected on 27 August and 22 September were negative for all viruses assayed.
DISCUSSION
We report on a massive outbreak of acute gastroenteritis in Podgorica, Montenegro. While 1699 cases of acute gastroenteritis were reported to IPH from 23 August to 7 September, the outbreak may have affected over 40 000 individuals. However, as August is usually the time when many inhabitants of Podgorica embark on their holidays, there is significant migration out of the city during this month and therefore the outbreak may have affected about 10 000–15 000 people. Epidemiological and microbiological investigations suggested that the source of this outbreak was faecally contaminated municipal drinking water. Norovirus was identified as a likely causative agent of the outbreak, but involvement of additional pathogens cannot be excluded. The identification of rotavirus and adenovirus in some patients might be related to the outbreak or may constitute background cases.
In the case-control study, illness was significantly associated with drinking unboiled, municipal drinking water, while drinking bottled water was protective. None of the study participants who denied consumption of municipal water in the week before the outbreak started reported having had symptoms of acute gastroenteritis. We did not find an association with the amount of unboiled tap water consumed as has been reported in other outbreaks associated with drinking water [Reference Goldstein12]. This may suggest considerable contamination of the drinking water supply which was enough for those people who drank a smaller number of glasses of unboiled, chlorinated drinking water to still be exposed and infected. Alternatively, a plausible explanation could be inaccurate exposure assessment because the number of glasses consumed during the week before the outbreak is likely to represent only a rough estimate and not exact recall.
Some norovirus activity is present all year round involving the discharge of different virus strains into urban sewage. The isolation of not less than six distinct norovirus genotypes from the faecal samples analysed implies a heavily contaminated source of infection, e.g. sewage contamination of food or drinking water [Reference Gallimore13, Reference Le Guyader14]. The absence of detectable norovirus in several of the faecal samples might be explained by the late sample collection in the course of illness for some cases, possible low viral shedding by some subjects and/or inadequate storage and transport conditions for samples [Reference Gray15]. The finding that some of the samples proved positive only by one of the two RT–PCR methods used is also consistent with a low viral load in stools, in addition to possible different recognition of individual target sequences by distinct primers. Due to the high genetic diversity of the norovirus genome, no single primer set is considered suitable for detecting all viral genotypes, and the use of other techniques in addition to RT–PCR is normally adopted to increase the chance of diagnosis. Waterborne outbreaks attributed to norovirus are usually difficult to recognize. Typically, only extensive outbreaks, which usually prompt thorough investigations, are attributed to water as a possible source of infection [Reference Hewitt16, Reference Maunula, Miettinen and von Bonsdorff17].
Investigation of the municipal water supply and its quality control revealed multiple weaknesses. At certain points in the municipal water supply, testing of final drinking water revealed total chlorine levels to be zero with numbers of coliforms exceeding satisfactory drinking-water quality levels. Furthermore, the contact time for chlorination and the subsequent residual chlorine concentration was unknown as there was no holding tank for any of the water sources. There was a history of water pump failures in the month before the outbreak, including 22 August, the day before the number of cases began to increase. The subsequent draining of the water pipes could have created a drop in water pressure with subsequent ingress of faecally contaminated water or raw sewage into the drinking-water distribution system, resulting in the outbreak [Reference Jakopanec18, Reference Nygard19]. The presence of several genotypes of norovirus in this outbreak adds credence to this theory since raw sewage has previously been the cause of norovirus infections and outbreaks [Reference Nenonen20, Reference Iwai21]. At the same time, this argues against contamination of the water directly at the source, as it is unlikely that such a wide spectrum of norovirus genotypes would enter the source water.
The case-control study is subject to several biases. For example, differential recall may have affected validity of the study if cases, especially those having been alerted by rumours or the media, presumed that municipal drinking water was a possible source of infection. Cases could then be more likely to report drinking unboiled tap water than controls. The delay between exposures and their assessment might have led to recall difficulties equally affecting cases and controls, thereby biasing the results towards the null value. The proportion of cases and controls invited to participate in the study were not recorded in a systematic manner. Extrapolating the results of the case-control study as well as of the survey of ill neighbours from the North-western districts to the entire city requires some faith. However, all districts of Podgorica were affected and there were no marked increase in reports of acute gastroenteritis noted in any other region of Montenegro. Thorough virological examination was limited to a relatively small number of faecal samples because sample expedition was logistically difficult and costly. However, we do not believe that analysis of more samples would have greatly benefited the investigation or altered the recommendations herein. A mechanism for sending appropriate human and environmental samples abroad should be established, formalized and duly funded. Procedures should be considered to test the efficiency of logistic and administrative arrangements envisaged.
The investigations resulted in formulation of recommendations to national authorities pertaining to outbreak management, prevention of similar outbreaks in the future and fostering of an inter-sectoral approach. Upon suspicion of drinking water contamination, procedures should be in place to facilitate timely action to control the public health risk. We therefore proposed several short- and long-term recommendations to IPH and the Ministry of Health including increased monitoring of source water and final water and the introduction of automatic and permanent measuring devices for free, residual chlorine. Furthermore, as detection of the outbreak was timely and municipal drinking water was almost immediately suspected as the source of infection, the IPH and Water Authorities were quick to increase the chlorine concentration at the main water-treatment works. However, as problems with chlorine dosing had been previously reported, another option for the authorities would have been to issue a boil water notice (or a restriction of consumption) [Reference Harrison22]. Moreover, the epidemic curve demonstrates that hyperchlorination did not result in a decrease of cases to background levels for quite some time. This may have been due to prolonged or intermittent presence of viruses in the municipal water system, to secondary spread, increased visits to physicians by people with milder illness, or a combination of these factors. The importance of inter-sectoral collaboration cannot be understated when investigating incidents or outbreaks [Reference Cassady23]. Collaborative preventive health and water-system protection activities should receive priority attention for implementation in high-, middle- and low-income countries. A multiple barrier approach should be developed for source, treatment, efficient distribution, monitoring and responding appropriately to breaches in quality [Reference Huck and Coffey24]. For this approach to be successful it is imperative that all these elements are maintained effectively by obligating the individual performances of statutorily appointed water companies, local authorities, primary-care departments, local and national health protection teams to meet best practice criteria.
ACKNOWLEDGEMENTS
We thank J. F. Munoz of the French Food Safety Agency for technical expertise. We acknowledge the contributions of Veselinka Beatović, Božidarka Rakočević, Sanja Medenica, Maja Milanović, Anton Đuravčaj, Jadranka Mahmutović, Dušica Novović, Jelena Fatić, Radmila Škatarić, Vjera Radulović, Fahrija Stanković and Ivan Đurović for the field investigation. We thank J. M. Cowden, Health Protection Scotland, for critically reviewing the manuscript.
DECLARATION OF INTEREST
None.






