Antibiotic usage has exponentially increased in developing countries, especially in India over the last decade. In 2010, India was the world’s largest consumer of antibiotics for human health followed by China and the United States.Reference Boeckel, Gandra and Ashok1 The increase in antibiotic use in India has been enabled by rising incomes, availability of cheaper generic antibiotics, unregulated over-the-counter pharmacy dispensation, indiscriminate antibiotic use in livestock, and inappropriate antibiotic use in health care.Reference Laxminarayan, Matsoso and Pant2 This situation is further aggravated by inadequate public health and infection control measures.Reference Laxminarayan, Matsoso and Pant2 Unfortunately, this increased antibiotic consumption has exerted selective pressure leading to emergence of resistant organisms.Reference Summers3 Antibiotic consumption is the driving factor for antimicrobial resistance, and a coordinated effort is needed to optimize antibiotic usage for both humans and animals, to prevent the transmission of resistant organisms, and to decrease environmental decontamination.4 Clear and increasing evidence indicates that the first step to preventing the global spread of antimicrobial-resistant bacteria is the reduction of antimicrobial consumption.Reference Čižman, Srovin and Pokorn5 Antimicrobial resistance poses a grave threat to the potential gain achieved in reducing mortality related to infections in the previous centuryReference Armstrong, Conn and Pinner6 by rendering all available antibiotics ineffective against the resistant organisms. Because there are fewer antibiotics in the pipeline for the foreseeable future, antimicrobial stewardship programs (ASPs) have emerged as a key strategy in combating antimicrobial resistance.
The absence of robust ASPs has led to rampant antimicrobial misuse, which in turn has led to antimicrobial resistance in most high-volume tertiary-care centers in India, impacting patient outcomes. Current stewardship efforts in India are few and far between; there is a significant dearth of trained personnel in most hospitals in addition to the absence of facility-specific antimicrobial guidelines and infection control measures.Reference Dellit, Owens and McGowan7–Reference Arnold, McDonald, Smith, Newman and Ramirez9 The infectious diseases (ID) specialist physician training programs started 8 years ago, and only ~10 such programs are active in the country today. Although the doctoral-level training programs for “clinical pharmacist” started 10 years ago, no specialty ID/ASP training program is currently available in the country, which has hindered the development and implementation of robust ASP programs in the country.
Methods
Study design and setting
Unlike in developed countries where robust ASPs have regulatory support, ASP activities in India remain in a nascent stage. The very concept of an ID clinical pharmacist is nonexistent in India. Even in our institution, there was no integrated, multidisciplinary ASP team as recommended, and ASP activities were restricted to antimicrobial resistance monitoring and publishing of hospital antibiograms and antibiotic guidelines. At the time of the study, the role of the hospital infection control committee (HICC) consisted of surveillance monitoring for hospital acquired infections (HAIs) by infection control nurses supervised by a clinical microbiologist along with hand hygiene audits. A hospital infection control manual was developed in 1996, adult antibiotic guidelines have been in effect since 2000, and we have used an antibiogram since 2011. All of these are updated regularly. Formerly, there was no formal ASP team in our hospital. We have had a formal ID training program run by qualified ID physicians, who have been actively involved in HICC, for the last 10 years only. We decided to assess the impact, feasibility, and effectiveness of an ID physician–driven antimicrobial stewardship strategy in our high-volume Indian setting. We chose a “prospective audit and feedback” strategy over restriction of formulary because it is conciliatory and engages and educates the primary treating team on ASP, rather than the new team impinging on their autonomy. In addition, we wanted to obtain data to present to the administration to advocate for the creation of a formal ASP team and program. In the absence of government regulations preventing overuse or abuse of antibiotics, it is often difficult to convince physicians of the harms of inappropriate antibiotic use. This prospective cohort study was carried out as a pilot initiative to determine whether it was feasible to implement antimicrobial stewardship in 2 of 9 acute-care areas of a 2,858-bed tertiary-care hospital in southern India that cares for up to 8,800 outpatients daily and 500,000 inpatients on an annual basis. The intensive care units (ICUs) have a total of 98 beds with patients from medical units, surgical units, and subspecialties, including bone marrow and solid organ transplant units that use large volumes of broad-spectrum antibiotics. We chose to conduct this study in 2 intensive care settings (medical and surgical ICUs) where antibiotic utilization is higher than in other ICUs.
The institutional review boards of Christian Medical College, Vellore (IRB no: 9747, dated 18.1.2015) and Henry Ford Health System approved the study. A waiver of consent was obtained because we studied prescription behavior of the treating physicians before and after consultation with the ID physician without any procedures being performed on the patient. The study period consisted of 18 months from January 2016 to July 2017 in 3 phases: baseline, intervention, and follow-up. Each phase consisted of 6 months with no washout period between the phases.
Identification of study participants and eligibility criteria
The participants were selected from the medical and surgical ICUs, managed by ICU staff in consultation with the primary treating team, who remained the same throughout the study. Any patient admitted to medical or surgical ICUs, aged 15 years or older, on study antimicrobials >48 hours and continued thereafter, were identified and recruited into the study. The chosen study antimicrobials were fluoroquinolones, β-lactam/β-lactamase inhibitor combinations, third- and fourth-generation cephalosporins, carbapenems, linezolid, tigecycline, azithromycin, doxycycline, colistin, and vancomycin. All medical and surgical specialties were included in the study, and patients fulfilling the eligibility criteria were recruited from Monday through Saturday.
Data capture and evaluation of antimicrobial use
Medical records of the patients were reviewed by the research assistants, and necessary details including the demographic data, comorbid conditions, culture and susceptibility reports, laboratory reports, antibiotic utilization, and the clinical outcome of the patients were obtained. For data collection, a standardized form was used. All data were collected initially on paper proformas and were later transcribed into an electronic database. A study number was assigned to each patient to maintain confidentiality. A study ID physician and a senior ID consultant were involved throughout the study. The study was divided into 3 phases. The baseline phase involved evaluation of antimicrobial therapy and appropriateness for the same without provision of any recommendations. During the intervention phase, the ICU team initiated an ASP consult for all eligible patients identified by the research assistant. During the ASP consult, the study ID physician provided a fairly detailed consultation to develop a rapport with the ICU team and the primary treating team because both were reticent to act on advice based solely on review of clinical and laboratory documents. We felt that this approach would be better accepted in the absence of an existing multidisciplinary team. Follow-up alerts regarding our recommendations were issued through electronic medical records; however, the final decision to act on the recommendations was left to the ICU or treating physician. The acceptance of our recommendations was recorded at the first 48-hour review. The changing clinical condition of the patient was monitored by the study team for a further 48-hour period, and relevant recommendations were provided by the study physician if necessary.
During the follow-up phase, we assessed the appropriateness of antibiotics without providing any recommendations, similar to the baseline phase, to determine whether there was a sustained post-intervention effect on the antibiotic prescription behavior among the ICU and treating teams.
Outcome measures
The primary outcome measure was days of therapy (DOT) with the study antimicrobials per 1,000 study patient days in the baseline and intervention phases. One DOT represents the administration of a single agent on a given day regardless of the number of doses administered or dosage strength. In this study, patient days included the day on which treatment with the antibiotic began through the stoppage of the drug or until hospital discharge.
Secondary outcomes included proportion of prescriptions with inappropriate antibiotic use, defined as inappropriate use in the absence of clinical indication, pathogen–antibiotic mismatch, unnecessary double coverage, or when the antibiotic was prescribed at a wrong dose, route, and/or frequency. Process measures included rates of de-escalation according to culture susceptibility and clinical evaluation, intervention rate (defined as the number of courses of therapy in which a modification is recommended divided by the total number of courses), and acceptance rate (calculated as the number of recommendations accepted divided by the number of recommendations made). In addition, we compared compliance with antimicrobial guidelines during the 3 phases. Patient-specific outcomes were the length of stay in the ICU and hospital, all-cause mortality, readmission rate to the ICU during the same admission, and unintended consequences (eg, adverse drug reactions, Clostridium difficile infections, and candidemia). Prevalence of multidrug-resistant organisms like methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Enterobacteriaceae (CRE), vancomycin-resistant Enterococcus (VRE), extended-spectrum β lactamases (ESBL), and vancomycin-resistant Staphylococcus aureus (VRSA) were also assessed.
Statistical approach
Frequencies and percentages were presented for all categorical outcomes. Days of therapy for study antimicrobials per 1,000 patient days were calculated for the baseline, intervention, and follow-up phases and were compared using a test for proportions with a Bonferroni correction where possible. For the comparison of length of ICU stay and hospital stay, we used the Kruskal-Wallis test because the distribution was not normal (which was assessed using a QQ plot and a histogram). The death rates and readmission rates were compared across the 3 study phases, adjusting for covariates using generalized linear models with the logistic link function. The duration of antibiotic was also compared across the study phases, adjusting for death and readmission with other comorbidities and confounders, using generalized linear models. P <.05 was considered statistically significant. We used SAS version 9.3 software (SAS Institute, Cary, NC) for analysis.
Results
Study population
During the baseline phase, 1,076 patients were admitted to the ICU, of whom 401 patients were enrolled. Similarly, 381 of 1,118 patients were enrolled in the intervention phase, and 379 of 1,144 patients were enrolled in the follow-up phase. The mean age, comorbidities, severity of illness, infectious versus noninfectious diagnoses, and primary source of infection were similar in all 3 study groups. Surgical patients predominated in all 3 phases (63.7%). Most patients had been admitted to general surgery, hematology, orthopedics, nephrology, urology, obstetrics and gynecology, followed by neurosciences. Among the patients recruited, admissions due to an infectious cause alone accounted for 21.4% of cases. Noninfectious causes accounted for 29.9% of cases, and the remaining cases (48.7%) were a combination of both. Among the infectious causes, intra-abdominal infections (18.7%) were common, followed by skin and soft-tissue infections (15.9%). Community-acquired infections accounted for 55.6% of the infection cases (Table 1).
Table 1. Baseline Characteristics of Patients Enrolled During the Baseline, Intervention, and Follow-Up Phases
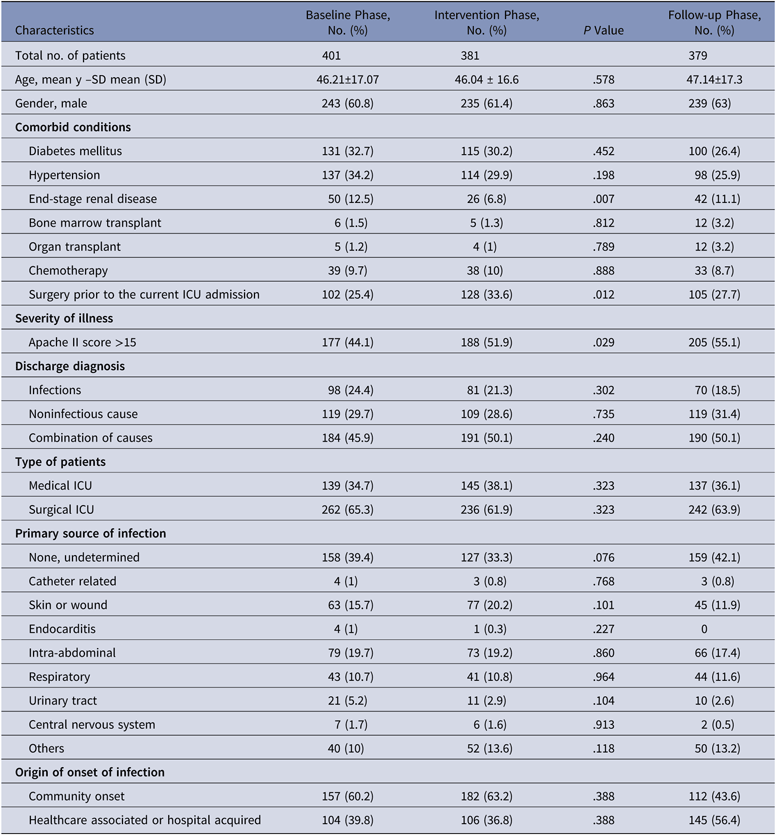
Note. SD, standard deviation; ICU, intensive care unit.
Antimicrobial use
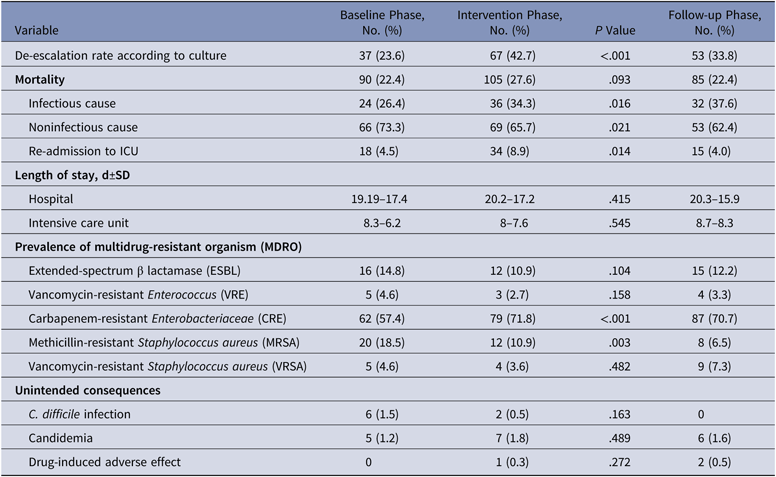
The antibiotics most commonly used were carbapenems, colistin, and β-lactam/β-lactamase inhibitors in all 3 groups. Among the antibiotics used to cover gram-positive organisms, teicoplanin followed by clindamycin and then vancomycin were most often utilized. The primary outcome, DOT per 1,000 patient days for study antimicrobials was 831.5 in the baseline phase. The DOT for the intervention phase was significantly lower at 717.0 (P < .0001), and this effect was sustained in the follow-up phase (DOT, 713.6). Among the study antimicrobials, antibiotic use was significantly lower in the intervention group versus the baseline group for quinolones, carbapenems, colistin (polymyxin E), and azithromycin (Table 2). The use of β lactam/β-lactamase inhibitor combinations increased in the intervention group compared to the baseline group. Total antimicrobial use, including study and nonstudy antimicrobials, was significantly lower in the intervention group versus the baseline group (987 vs 1,201.7 DOT; P < .0001). Even among nonstudy antimicrobials, use significantly declined for cotrimoxazole, metronidazole, teicoplanin, clindamycin, and aminoglycosides in the intervention phase compared with the baseline phase. The rates of compliance with hospital-based antibiotic guidelines increased steadily; they were 19.5% in the baseline phase, 21.8% in the intervention phase, and 33.2% in the follow-up phase. The rates of inappropriate use of antibiotics declined steadily; they were 77.6%, 70.3%, and 58.6% in the baseline, intervention, and follow-up phases, respectively. The main reason for inappropriate antibiotic use was that they were not clinically indicated (38.6%), followed by the unnecessary double coverage for anaerobes offered by second antibiotics (19.8%). The rate of de-escalation according to culture susceptibility was significantly higher in the intervention group compared to the baseline group (42.7% vs 23.6%; P < .001), suggesting a definite trend with regard to the judicious use of antibiotics (Table 3).
Table 2. Overview of Antibiotic Therapy at Day 3 and Justification by the Infectious Diseases Team
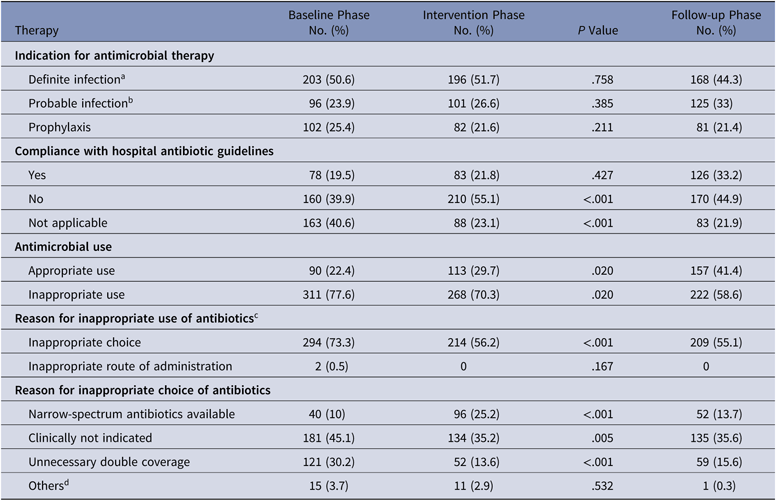
a Definite infection is defined as a clinically documented infection with microbiological confirmation.
b Probable infection is defined as clinically documented infection without microbiological confirmation.
c The percentages do not add up to 100% due to overlap.
d Pathogen–antibiotic mismatch.
Table 3. Primary Outcome: Days of Therapy (DOT) of Antimicrobials in the Baseline, Intervention, and Follow-Up Phases
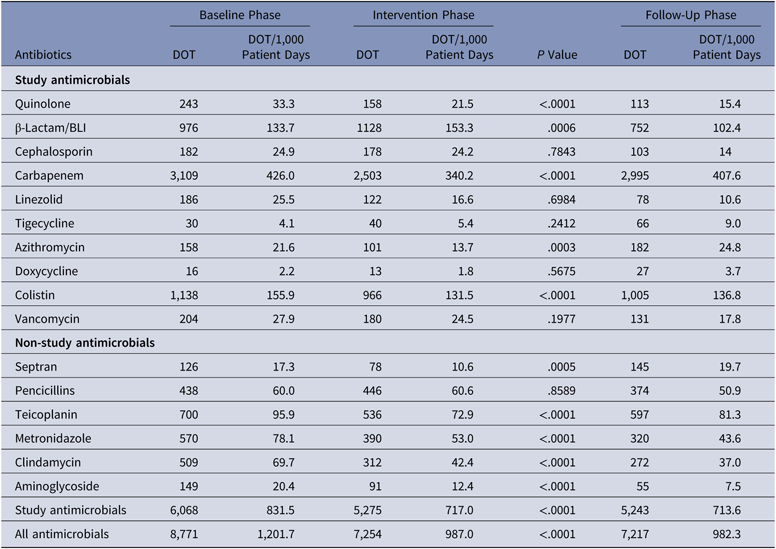
Note. BLI, β-lactamase inhibitor.
In the intervention phase, the recommendations made were either to stop, to modify and de-escalate, or to continue the prescribed antimicrobial therapy. In total, 381 patients were reviewed, and recommendations to modify therapy were made for 268 patients based on inappropriate therapy. Hence, the intervention rate was 70.3%. Overall, the recommendations by the ID team were accepted in 60.7% of cases. Antibiotic recommendations given by the ID team were fully followed in 182 cases (47.8%), partially followed in 49 cases (12.9%), and not followed in 137 cases (35.7%). Specifically, de-escalation, recommended by the ID team in 97 cases, was followed in 41 cases (42.2%). Discontinuation, recommended in 185 cases, was followed in 82 cases (44.3%). Ending unnecessary double coverage, recommended in 31 cases, was done in 20 cases (64.5%). Modification according to susceptibility, recommended in 9 cases, was done in 2 cases (22.2%). Continuing the same treatment was recommended in 103 cases and was followed in 97 cases (94.1%) (Table 5).
Table 4. Secondary Outcomes at Discharge
Note. SD, standard deviation; ICU, intensive care unit.
Table 5. Acceptance of Recommendation Given by the Infectious Disease Physician During the Intervention Phase
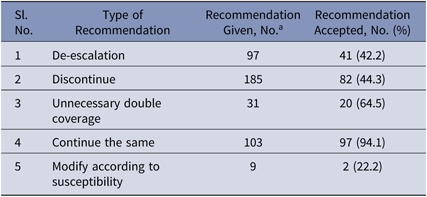
a Numbers will not add up to the total number of patients (n = 381) because single patient may have received multiple recommendations.
Antimicrobial resistance
The overall prevalences of multidrug-resistant organisms (MDROs) included in the study (CRE, ESBL, MRSA, VRE, and VRSA) were 26.9% in the baseline phase, 28.8% in the intervention phase, and 32.4% in the follow-up phase. The organism with the highest prevalence was carbapenem-resistant Enterobacteriaceae (CRE), which comprised 57.4% of MDROs in the baseline phase, 71.8% in the intervention phase, and 70.7% in the follow-up phase (Table 3).
Clinical outcomes
Mortality rates in the baseline, intervention, and follow-up phases were 22.4%, 27.6%, and 22.4%, respectively. The respective lengths of stay in the hospital in the baseline, intervention, and follow-up phases were 19.19±17.4 days, 20.2±17.2 days, and 20.3±15.9 days. The respective lengths of stay in the ICU were 8.3±6.2 days, 8±7.6 days, and 8.7±8.3 days. There was no significant difference in the mortality (P = .093), length of stay in hospital (P = .415), or length of ICU stay (P = .545) between the baseline and intervention phases (Table 3). The readmission rates were 4.5%, 8.9%, and 4.0% respectively during the baseline, intervention, and follow-up phases.
The unintended consequences of antibiotics were also considered. Clostridium difficile infection was identified in 1.5% of cases in the baseline group and 0.5% of cases in the intervention group. Candidemia occurred in 1.2% of cases in the baseline group, 1.8% of cases in the intervention group, and 1.6% in the follow-up group. Drug-induced adverse effects occurred in 0.3% of cases in the intervention group and 0.5% of cases in the follow-up group. We found no significant difference in the rates of C. difficile infection (P = .163), candidemia (P = .489), or adverse drug reactions (P = .272) during the intervention and follow-up phases (Table 4).
Discussion
This pilot study is the first in India to examine the impact of implementation and utility of an “ID physician–driven prospective feedback and review” strategy of antimicrobial stewardship in 2 intensive care settings in a high-volume tertiary-care hospital in South India.
We noted a marked decrease in the use of reserve antibiotics like carbapenems, colistin, and teicoplanin, which was similarly reported in other studies.Reference Chang, Chen and Lin10, Reference Karanika, Paudel, Grigoras, Kalbasi and Mylonakis11 In contrast, the use of β lactam/β-lactamase inhibitor combinations went up in the intervention group as compared to the baseline and we attribute this to the de-escalation from the carbapenems and polymyxins.
We found that 70% of the total antibiotic prescriptions were inappropriate, which is higher than the 30% rate reported in studies conducted in high-income settings.Reference Morrill, Caffrey, Gaitanis and LaPlante12 Our finding suggests high inappropriate use of antibiotics in India. The main reason for inappropriate antibiotic use was the unnecessary use of antibiotics in the absence of a clinical infection or indication, which is similar to studies in high-income countries.Reference Morrill, Caffrey, Gaitanis and LaPlante12 It was easier to convince the primary treating team to discontinue unnecessary double coverage in the intervention phase than to discontinue antibiotics when they were clinically not indicated (Table 5).
The acceptance of our recommendations was 60% which is comparable to most previous studies in high-income settings, which report acceptance rates in the range of 60%–70%.Reference Morton, Curzake, Parente, Morrill, Gaitanis and Laplante13, Reference Chung, Wu, Yeo, Chan and Hsu14 During the intervention phase, recommendations made through electronic medical record notes (EMR) alone did not lead to acceptance of recommendations. However, active dialogue and constant interaction with the in-house ICU staff and primary treating team along with 2 subsequent 48-hour visits by the ID team (to monitor the patient’s progress after the recommendations were given) seemed to ensure better acceptance. Dialogue and discussion akin to a full ID consultation between the study ID physician and the ICU staff was necessary to establish trust and rapport. Moreover, ID consults took the opportunity to educate various teams about and create awareness of the ASP. Overall, we found that prospective audit and feedback in the intervention phase reduced antibiotic utilization during both the intervention and follow-up phases, as reported in similar studies.Reference Lesprit, de Pontfarcy and Esposito-Farese15, Reference Tamma, Avdic and Keenan16 In our study, this effect was sustained in the follow-up phase to a higher degree, however.
Compliance to institution-specific antibiotic guidelines showed marginal improvement in the intervention phase and significant improvement in the follow-up phase. In 28.5% of cases, antibiotic guidelines were not applicable, suggesting a need for comprehensive national and institution-specific antibiotic policy guidelines based on the local antibiogram.
The prevalence of MDROs was almost 30% across all phases of the study; most were CRE (66.3%). This prevalence likely led to the increased use of reserve antibiotics like carbapenems and colistin: the ESBL and CRE rates were high. There was no significant reduction in the use of vancomycin and linezolid with our intervention, probably because the background MRSA (11.9%) and VRSA (5.1%) infection rates were low. We did not observe any significant reduction in resistance rates, which is expected because this study targeted only 2 specialized areas, not the entire hospital. Notably, in spite of the higher prevalence of CRE in the intervention phase versus the baseline phase (71.8% vs 57.4%), our de-escalation rate was high, suggesting that the intervention was indeed remarkably significant. In addition, an intervention of 6 months is too limited a period of time to impact resistance rates significantly.
Mortality seemed higher in the intervention phase against baseline but was statistically insignificant (P = .093). There were no significant differences in the length of hospital stay (P = .087) or ICU stay (P = .138) between the baseline and intervention phases. Although the readmissions in the intervention phase were significantly higher, this was due to sicker patients with higher APACHE II scores (P = .035) and the high CRE rate (71.8%). Although our study intervention led to decreased antimicrobial use, there was no significant survival benefit,Reference Okumura, da Silva and Veroneze17, Reference Brink, Messina and Feldman18 unlike results reported in high-income countries.
When considering the unintended consequences of antibiotic use, our Clostridium difficile rates were very low (0.6%), even with high antibiotic consumption when compared to other studies, which showed a rate of ~30%.Reference Baur, Gladstone and Burkert19 Thus, C. difficile as an outcome measure probably needs to be re-evaluated in the Indian context because the rate is much lower than in developed countries.
This study has several limitations. It was a quasi-experimental study without an interrupted time series and washout period due to logistic reasons, and it was planned as a short pilot project to be followed by full-scale implementation if feasible. The study was also limited by lack of information on compliance with infection prevention practices; however, there were no changes in infection prevention policies over the study period. The study was also limited to an 18-month period, making assessments of changes in resistance impossible. Resistance in the present study is also compounded by factors outside the control of a hospital program such as the outpatient use of antimicrobials and antibiotic use in animal feed and aquaculture.
Our study demonstrated that a trained ID physician-led ASP was labor intensive but rewarding, even with increased patient numbers and complex critical patients in the ICU setting. In our large tertiary-care hospital, this pilot initiative had a measure of success in reducing antimicrobial overuse in an acute-care setting with high antibiotic utilization. In our program, antibiotic use was closely monitored, and we achieved a balance between limiting the availability of antibiotics and ensuring timely treatment for severe infections. Cross-disciplinary collaboration and education were essential components of our program, proving that this is indeed feasible in a LMIC setting. Although our study demonstrated that an ID physician–driven ASP can bring about significant reduction in the use of reserve antibiotics, a multidisciplinary team approach that builds the capacity of clinical pharmacists is required to implement a full-scale workable and sustainable ASP model in India. The biggest achievement of our study was sensitizing the hospital management and other departments to the emerging threat of antimicrobial resistance and the immediate need to act before it is too late.
Acknowledgements
Merck Sharp & Dohme (MSD)
Financial support
The Merck Foundation provided funding for the study; however, they had no role in study design, implementation, data collection, data interpretation, or writing of the report.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.









