Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by Crossref.
Wattengel, Bethany A.
Sellick, John A.
and
Mergenhagen, Kari A.
2020.
Outpatient antimicrobial stewardship: Optimizing patient care via pharmacist led microbiology review.
American Journal of Infection Control,
Vol. 48,
Issue. 2,
p.
189.
Ramakrishnan, Aditi
and
Patel, Payal K.
2020.
How Far We’ve Come, How Far We Have to Go: a Review of Advances in Antimicrobial Stewardship in the Veterans Health Administration.
Current Treatment Options in Infectious Diseases,
Vol. 12,
Issue. 3,
p.
275.
Greene, Matthew H.
Nesbitt, Whitney J.
and
Nelson, George E.
2020.
Antimicrobial stewardship staffing: How much is enough?.
Infection Control & Hospital Epidemiology,
Vol. 41,
Issue. 1,
p.
102.
Lowery, James L.
Alexander, Bruce
Nair, Rajeshwari
Heintz, Brett H.
and
Livorsi, Daniel J.
2021.
Evaluation of antibiotic prescribing in emergency departments and urgent care centers across the Veterans’ Health Administration.
Infection Control & Hospital Epidemiology,
Vol. 42,
Issue. 6,
p.
694.
Gillespie, Chris
Sitter, Kailyn
McConeghy, Kevin W.
Strymish, Judith
Gupta, Kalpana
Hartmann, Christine W.
and
Borzecki, Ann M.
2023.
Facilitators and Barriers to Verifying Penicillin Allergies in a Veteran Nursing Home Population.
The Journal of Allergy and Clinical Immunology: In Practice,
Vol. 11,
Issue. 9,
p.
2848.
Jones, DeShauna
Marra, Alexandre R.
Livorsi, Daniel
Perencevich, Eli
and
Goto, Michihiko
2023.
Perceptions of an automated benchmarking dashboard for antimicrobial stewardship programs among antimicrobial stewards within the veterans’ health administration: a multicenter qualitative study.
Antimicrobial Stewardship & Healthcare Epidemiology,
Vol. 3,
Issue. 1,
Livorsi, Daniel J.
Branch-Elliman, Westyn
Drekonja, Dimitri
Echevarria, Kelly L.
Fitzpatrick, Margaret A.
Goetz, Matthew Bidwell
Graber, Christopher J.
Jones, Makoto M.
Kelly, Allison A.
Madaras-Kelly, Karl
Morgan, Daniel J.
Stevens, Vanessa W.
Suda, Katie
Trautner, Barbara W.
Ward, Michael J.
and
Jump, Robin L.P.
2024.
Research agenda for antibiotic stewardship within the Veterans’ Health Administration, 2024–2028.
Infection Control & Hospital Epidemiology,
p.
1.
Smith, Matthew
Crnich, Chris
Donskey, Curtis
Evans, Charlesnika T.
Evans, Martin
Goto, Michihiko
Guerrero, Bernardino
Gupta, Kalpana
Harris, Anthony
Hicks, Natalie
Khader, Karim
Kralovic, Stephen
McKinley, Linda
Rubin, Michael
Safdar, Nasia
Schweizer, Marin L.
Tovar, Suzanne
Wilson, Geneva
Zabarsky, Trina
and
Perencevich, Eli N.
2024.
Research agenda for transmission prevention within the Veterans Health Administration, 2024–2028.
Infection Control & Hospital Epidemiology,
p.
1.



(See introductory commentary by Livorsi et al, pages 186–188.)
Antimicrobial use is a key contributor to increasing antimicrobial resistance.Reference Livermore 1 Antimicrobial stewardship has been shown to decrease inappropriate antimicrobial use and, in turn, may reduce antimicrobial resistance and Clostridium difficile infection (CDI).Reference Barlam, Cosgrove and Abbo 2 Antimicrobial stewardship can also improve clinical outcomes for patients.Reference Schuts, Hulscher and Mouton 3
The Veterans Health Administration (VHA) is a leader in promoting antimicrobial stewardship.Reference Wagner, Filice and Drekonja 4 – Reference Kelly, Jones and Echevarria 7 Since 2011, the VHA National Antimicrobial Stewardship Taskforce (ASTF) has provided guidelines and resources for stewardship implementation at individual VHA facilities. In 2014, the VHA mandated that every facility develop and maintain an antimicrobial stewardship program (ASP). The VHA ASP Directive tasked every VHA facility to have an ASP policy, to conduct an annual evaluation of ASP activities, and to have a provider and a pharmacist champion.Reference Chou, Graber and Jones 6 There were no requirements for facilities to adopt any specific set of ASP activities, allowing individual facilities to decide which elements to implement based on their own focus areas and available resources. This mandate was among the first in the United States, preceding similar directives from the Joint Commission and the Centers for Medicare and Medicaid Services. 8 , 9 Recently, VHA investigators have partnered with the Centers for Disease Control and Prevention (CDC) to make use of novel antimicrobial usage metrics, including the standardized antimicrobial administration ratio (SAAR). They have also utilized the robust VA Corporate Data Warehouse (CDW) to determine the effectiveness of ASPs, to evaluate outcomes, and to identify high-impact targets.Reference Chou, Graber and Jones 6 , Reference Kelly, Jones and Echevarria 7 , Reference Jones, Huttner and Madaras-Kelly 10 – Reference Jones, Sauer and Jones 12
As the largest integrated healthcare system in the United States, the VHA provides a unique opportunity to study strategies to improve antimicrobial prescribing and the effectiveness of these interventions. Herein, we describe the proceedings from a multidisciplinary conference focused on developing an agenda for antimicrobial stewardship research in inpatient and outpatient healthcare settings within the VHA (long-term care is described separately, see commentary by Evans CT, pages 210–213). The methods are described elsewhere (see preceding editorial). The research targets were designed specifically for health services researchers within the VHA, but our findings also have implications more broadly in private sector healthcare settings (Table 1).
RECOMMENDATIONS
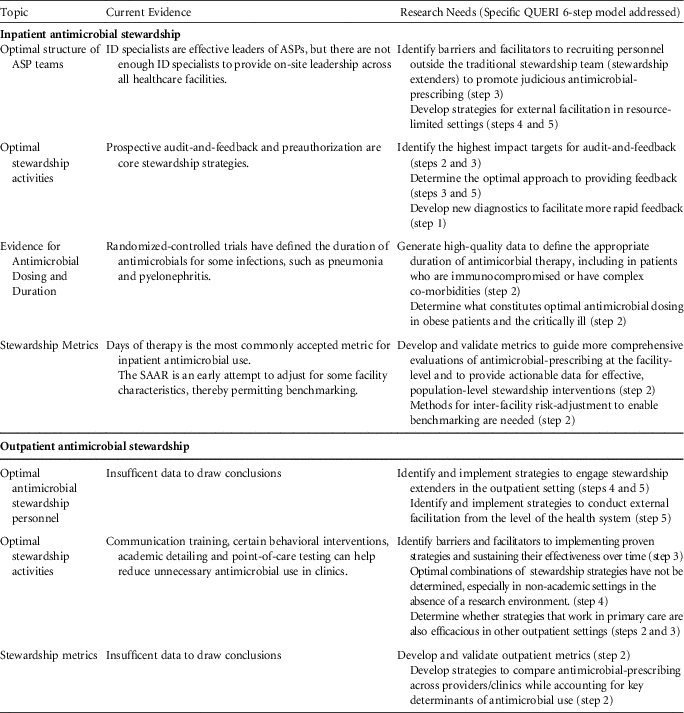
Inpatient Antimicrobial Stewardship
Optimal structure of ASP teams
Antimicrobial stewardship programs led by infectious disease (ID) specialists (physicians and/or pharmacists) have been successful at reducing unnecessary antimicrobial use and in improving appropriate use.Reference Barlam, Cosgrove and Abbo 2 , Reference Chou, Graber and Jones 6 , 13 However, due to staffing limitations, the implementation of stewardship across the continuum of health care is not feasible if an ID specialist must be the leader of every program. Many hospitals and nonacute residential care settings (eg, long-term care) lack and/or cannot afford ID specialists to assist with stewardship. Even at hospitals that have ID specialists involved in stewardship, limitations in both resources and time prevent routine intervention on every antimicrobial prescribing decision.Reference Echevarria, Kelly, Morreale, Neuhauser and Roselle 14
Future studies should identify barriers and facilitators to recruiting personnel outside the traditional stewardship team in the promotion of judicious antimicrobial prescribing. Depending on the clinical setting, these stewardship extenders may include non-ID pharmacists, hospitalists, and other ancillary staff, such as nursing.Reference Yam, Fales, Jemison, Gillum and Bernstein 15 – Reference Olans, Olans and DeMaria 17 In resource-limited settings, the use of telemedicine or information technology may provide opportunities for external facilitation.Reference Barlam, Cosgrove and Abbo 2 , Reference Stenehjem, Buckel and Jones 18 For example, local personnel at a resource-limited hospital could interact with an ID specialist based at a different location. Infectious diseases physicians have provided HIV training to primary care and rural locations through the VA Specialty Care Access Network-Extension for Community (SCAN-ECHO) program.Reference Moeckli, Stewart and Ono 19
Optimal stewardship activities
Prospective audit-and-feedback and preauthorization are both effective strategies for antimicrobial stewardship within inpatient settings.Reference Barlam, Cosgrove and Abbo 2 Recent evidence suggests that prospective audit-and-feedback may be more effective than preauthorization in decreasing overall usage.Reference Tamma, Avdic and Keenan 20
Audit-and-feedback is based on a set of core principles,Reference Brehaut, Colquhoun and Eva 21 and the effectiveness of this strategy is dependent on how the feedback is delivered.Reference Ivers, Jamtvedt and Flottorp 22 Future studies should identify which methods for providing feedback are most effective for antimicrobial stewardship.
Using audit-and-feedback, ASPs can address multiple aspects of antimicrobial prescribing, including empiric prescribing, antimicrobial dosing, de-escalation, duration of therapy, and intravenous-to-oral formulation conversion. It is unclear which of these targets has the greatest potential impact on key stewardship outcomes, such as preventing the emergence of antimicrobial resistance, reducing CDI, minimizing other adverse events, and improving clinical outcomes. ASPs have limited resources and effort should be directed toward activities with the greatest potential impact.
As much as possible, stewardship processes should be incorporated into the daily workflow of frontline prescribers.Reference Barlam, Cosgrove and Abbo 2 Clinical decision support systems (CDSSs) may be helpful in promoting thoughtful prescribing at the point of care, particularly by informing empiric prescribing decisions and prompting prescribers to de-escalate or discontinue therapy.Reference Forrest, Van Schooneveld, Kullar, Schulz, Duong and Postelnick 23 , Reference Holstiege, Mathes and Pieper 24 Further research on CDSSs should address not only the technical aspects of implementation but also the optimal methods for presenting complex data and antimicrobial recommendations in an understandable way.Reference Filice, Drekonja and Thurn 25
Many ASPs have partnered with microbiology labs to leverage rapid diagnostics and biomarkers to improve antimicrobial prescribing.Reference Timbrook, Morton, McConeghy, Caffrey, Mylonakis and LaPlante 26 Not only must these tests provide accurate and actionable results, but data must be standardized and results communicated effectively to change prescriber behavior.Reference Timbrook, Morton, McConeghy, Caffrey, Mylonakis and LaPlante 26 Developing better novel diagnostic tests and incorporating these tests into ASP efforts has great potential, because diagnostic uncertainty continues to be a major obstacle to appropriate antimicrobial use, especially in complex, dynamic environments such as critical care.
Evidence for antimicrobial dosing and duration
Standard antimicrobial doses are inadequate to meet pharmacokinetic and pharmacodynamic (PK/PD) targets in many patients.Reference Theuretzbacher 27 , Reference Roberts, Paul and Akova 28 Data on what constitutes optimal dosing are limited, especially for certain populations (eg, obesity, critically ill) and categories of antimicrobials. Further research is needed.
Randomized-controlled trials have defined duration of antimicrobials for some infections, such as pneumonia and pyelonephritis, but for many infections, the recommended duration is based on expert opinion (eg, cystitis in men).Reference Hayashi and Paterson 29 If clinical trials and comparative effectiveness studies can provide robust data on the optimal duration of therapy for normative patients, it would facilitate stewardship efforts to ensure that antimicrobials are administered for no longer than necessary. Studies on duration of therapy are also needed in immunocompromised and other patients with complex comorbidities, who were often excluded from the aforementioned trials.
Stewardship metrics
We are at an early stage in understanding how best to evaluate stewardship processes and how these processes influence antimicrobial prescribing and clinical outcomes. In acute-care hospitals, antimicrobial days per 1,000 days present is currently the most commonly accepted metric for antimicrobial use.Reference Barlam, Cosgrove and Abbo 2 However, reasonable targets for reduction have yet to be established. These targets would need to account for differences in patient populations as well as differences in types of antimicrobials prescribed (eg, broad- vs narrow-spectrum agents).
The VHA has been working with the CDC to implement SAAR ratios as part of the antimicrobial use module within the National Healthcare Safety Network (NHSN). 30 The SAAR is risk-adjusted for specific hospital characteristics and thereby represents an improvement over antimicrobial use metrics. However, the SAAR alone will not be sufficient in guiding more comprehensive evaluations at the facility level and in identifying institution-specific opportunities for improvement.Reference Moehring, Anderson and Cochran 31 SAARs do not provide data as to why antimicrobials are being prescribed (ie, use according to patient diagnosis) or how antimicrobials are being used (eg, number of patients exposed, rates and timing of de-escalation or overall duration of therapy). Additional metrics should provide actionable data to clinicians and stewardship teams on their antimicrobial prescribing behavior.
When making interfacility comparisons using metrics, inherent differences between hospitals must be considered. The SAAR attempts to adjust for some facility-level factors, such as facility bed size, number of intensive care unit beds, and facility medical school affiliation. 30 Adjustments may also have to be made for differences in case mix, infection prevention practices, and antimicrobial resistance rates. The best strategies to account for these key determinants of antimicrobial use have yet to be defined.
An overarching goal of stewardship is the reduction of antimicrobial resistance; therefore, a major priority includes determining which metrics of antimicrobial use best predict patient- or population-level changes in antimicrobial resistance. Of course, the impact stewardship has on antimicrobial resistance will always be confounded by how successfully infection prevention practices prevent patient-to-patient transmission of resistant organisms.
Stewardship programs should also quantify clinical outcomes, including infection-related deaths and infection-related readmissions.
Outpatient Antimicrobial Stewardship
Optimal antimicrobial stewardship personnel
Guidelines for acute-care ASPs recommend that leaders of stewardship strategies should be ID specialists.Reference Barlam, Cosgrove and Abbo 2 , 13 However, few ID specialists are available in the outpatient sector. Therefore, it is of utmost importance to engage stewardship extenders in outpatient care settings (eg, community pharmacists, public health departments). Patients are also an important component of outpatient stewardship; patient demand is a known predictor for inappropriate antimicrobial prescribing. Data on methods by which to engage these stakeholders are limited.Reference Gonzales, Steiner, Lum and Barrett 32 , Reference Little, Stuart and Francis 33 Research is also needed to identify strategies for external facilitation at the health system, payer, and/or federal level in the community.
Optimal stewardship activities
In the United States, most of the antimicrobial consumption occurs in the community,Reference Suda, Hicks, Roberts, Hunkler and Danziger 34 and at least 30% of prescribing is inappropriate.Reference Fleming-Dutra, Hersh and Shapiro 35 Stewardship strategies have been evaluated in the community, but most studies have focused on clinic-delivered interventions among primary care physicians. A systematic review demonstrated that communication training and point-of-care testing are effective in reducing unnecessary antimicrobial use in clinics, while education alone has failed to demonstrate a sustained benefit.Reference Drekonja, Filice and Greer 5 Certain behavioral interventions have also reduced inappropriate antimicrobial use for acute respiratory tract infections.Reference Meeker, Linder and Fox 36 Continued evaluation of the impact of other stewardship strategies, such as the role of clinical decision support systems, delayed prescribing, and audit and feedback measures should be conducted. A major priority is the identification of barriers and facilitators to implementing effective strategies across all outpatient settings and sustaining their effectiveness over time. Additionally, the generalizability of stewardship strategies across all outpatient care settings (eg, emergency departments, urgent care, and independent practices and across large healthcare networks) are unknown.
Most stewardship evaluations have focused on the outcome of unnecessary antimicrobial-prescribing among clinic-based primary care physicians. However, few evaluate appropriate antimicrobial selection (eg, first-line agents) when antimicrobials are indicated (eg, symptomatic bacteriuria). Evidence is also needed to support the impact of specific stewardship interventions in the community, especially for specialty physicians, nonphysician providers, resource-limited settings and outpatient care delivered outside of the clinic setting (eg, emergency departments, urgent care).
Outpatient parenteral antimicrobial therapy (OPAT) is time intensive and is frequently outside the purview of many outpatient stewardship initiatives. As a first step, we recommend studies of existing OPAT practices in VHA.
Stewardship metrics
Little evidence is available to identify optimal stewardship metrics for outpatient settings. While audits to assess overall use have been recommended,Reference Sanchez, Fleming-Dutra, Roberts and Hicks 37 it is unclear whether cases should be identified by International Classification of Disease, Tenth Revision (ICD-10) codes (syndrome-based approach) or antimicrobial prescriptions (agent-based approach). The impact of outpatient stewardship on antimicrobial resistance, CDI, and patient-centered outcomes has also not been definitively demonstrated, but research has suggested decreasing antimicrobial prescribing in outpatients has been associated with decreased resistance and CDI.Reference Dantes, Mu and Hicks 38 , Reference Seppala, Klaukka and Vuopio-Varkila 39
The use of metrics for peer-to-peer and interfacility comparisons will have to account for differences between patient panels, which could potentially include differences in comorbidities and community resistance rates. Like metrics for inpatient interfacility comparisons, the best strategies to account for these key determinants of antimicrobial use have yet to be defined.
Implementation of Antimicrobial Stewardship Across Inpatient and Outpatient Settings
Recent regulatory mandates both in the VHA and outside the VHA should facilitate the expansion of inpatient stewardship efforts. While VHA stewardship activities in outpatient care settings are increasing, these interventions remain limited.Reference Kelly 40 Data are not available on the prevalence of stewardship interventions in outpatient care settings in the private sector, but it is likely that these efforts also remain limited. Barriers and facilitators to broader implementation of outpatient stewardship have not been identified. In addition, optimal timing of outpatient stewardship interventions and the role of inpatient ASP teams to facilitate stewardship in outpatient healthcare settings remains unknown.
Achieving the research targets discussed above for both inpatients and outpatients should facilitate efforts of widespread stewardship implementation. For example, clarifying which stewardship activities are the most impactful should help to implement processes in settings with limited resources and across large healthcare networks. Factors that ensure sustainability and scalability over time need to be identified.
CONCLUSION
In conclusion, a multidisciplinary group of VHA experts identified several research targets for antimicrobial stewardship. Pursuit of research in these areas should improve health care for veterans and the nation while continuing to address the crisis of antimicrobial resistance. Addressing these knowledge gaps in the VHA has the opportunity to be far-reaching because healthcare systems in the private sector can apply what was learned in VHA to their own stewardship programs and initiatives. With a robust data source and a partnership between clinical care and research, the VHA provides a unique opportunity to evaluate the implementation and impact of a national antimicrobial stewardship policy.
TABLE 1 Assessment of Current Evidence and Recommendations for Research Needs in Antimicrobial Stewardship
NOTE. ID, infectious diseases; ASP, antimicrobial stewardship program; SAAR, standardized antimicrobial administration ratio.
ACKNOWLEDGMENTS
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Financial support: This work was supported in part by funding from the VA Health Services Research and Development (HSR&D) Service Center of Innovation (COIN) conference supplement for “Setting the Clinical Research Agenda for MDROs in VA” (grant no. CIN 13-412) and VA QUERI CARRIAGE program (grant no. IP1 HX001993-01A1).
Potential conflicts of interest: All authors report no conflicts of interest or financial disclosures relevant to this article.