Article contents
Validation of the SHEA/IDSA severity criteria to predict poor outcomes among inpatients and outpatients with Clostridioides difficile infection
Published online by Cambridge University Press: 30 January 2020
Abstract
To determine whether the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Clostridioides difficile infection (CDI) severity criteria adequately predicts poor outcomes.
Retrospective validation study.
Patients with CDI in the Veterans’ Affairs Health System from January 1, 2006, to December 31, 2016.
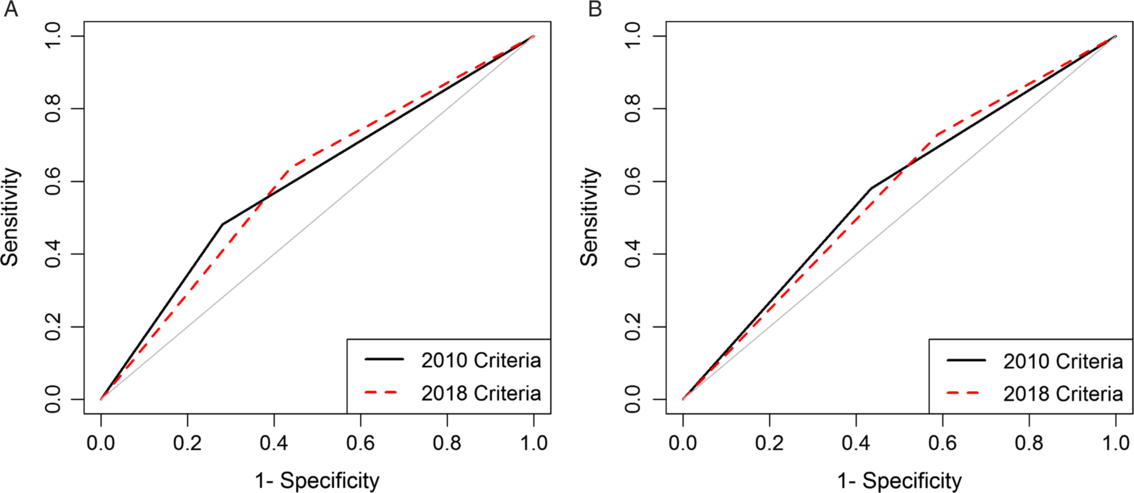
For the 2010 criteria, patients with leukocytosis or a serum creatinine (SCr) value ≥1.5 times the baseline were classified as severe. For the 2018 criteria, patients with leukocytosis or a SCr value ≥1.5 mg/dL were classified as severe. Poor outcomes were defined as hospital or intensive care admission within 7 days of diagnosis, colectomy within 14 days, or 30-day all-cause mortality; they were modeled as a function of the 2010 and 2018 criteria separately using logistic regression.
We analyzed data from 86,112 episodes of CDI. Severity was unclassifiable in a large proportion of episodes diagnosed in subacute care (2010, 58.8%; 2018, 49.2%). Sensitivity ranged from 0.48 for subacute care using 2010 criteria to 0.73 for acute care using 2018 criteria. Areas under the curve were poor and similar (0.60 for subacute care and 0.57 for acute care) for both versions, but negative predictive values were >0.80.
Model performances across care settings and criteria versions were generally poor but had reasonably high negative predictive value. Many patients in the subacute-care setting, an increasing fraction of CDI cases, could not be classified. More work is needed to develop criteria to identify patients at risk of poor outcomes.
- Type
- Original Article
- Information
- Creative Commons
- This work is classified, for copyright purposes, as a work of the U.S. Government and is not subject to copyright protection within the United States.
- Copyright
- © 2020 by The Society for Healthcare Epidemiology of America. All rights reserved
Footnotes
PREVIOUS PRESENTATION: Selected results from this manuscript were presented as a poster at IDWeek 2018 on October 4, 2018, in San Francisco, California.
References
- 4
- Cited by




