Introduction
Dementia refers to a “clinical syndrome of cognitive decline” that interferes with daily functioning and generally occurs alongside behavior and personality changes; the decline must not be the result of delirium or another condition (i.e. medical, neurological, or psychiatric) (Chertkow et al., Reference Chertkow, Feldman, Jacova and Massoud2013). The most common causes of dementia are Alzheimer's disease (50–75%), vascular dementia (20–30%), frontotemporal dementia (5–10%), and dementia with Lewy bodies (<5%) [Alzheimer's Disease International (ADI), 2014]).
The estimated global number of incident and prevalent cases of dementia in 2015 was 9.9 million and 46.8 million respectively (ADI, 2015). Worldwide, the age- and gender-standardized incidence of dementia among adults aged 60–64 was an estimated 3.9 per 1,000 person years, doubling with every 6.3 years of age to 104.8 per 1,000 person years among adults aged 90 and older (ADI, 2015). High income countries generally exhibited higher incidence than low and middle income countries, particularly across older age groups. Furthermore, the estimated age-standardized prevalence of dementia ranged from 1.3% among those aged 60–64 to 27.1% in those aged 90 and older in Central Europe (4.7% aged 60 and older), and from 2.2% among those aged 60–64 to 29.4% in those aged 85 and older in North Africa/Middle East (8.7% aged 60 and older) (ADI, 2015).
Original studies published over the last decade regarding time trends in dementia have reported mixed results in several different regions of the world. Key studies provide evidence of declining incidence in Rochester, United States (US) (Rocca et al., Reference Rocca2011) and Rotterdam, the Netherlands (Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012). Further studies indicate stable (Hall et al., Reference Hall2009) or declining dementia prevalence in a national US sample (Langa et al., Reference Langa2008), stable prevalence in a German sample (Doblhammer et al., Reference Doblhammer, Fink and Fritze2015), and declining prevalence in Zaragoza, Spain (Lobo et al., Reference Lobo2007) and regions of England and Wales (Matthews et al., Reference Matthews2013). In contrast, other research reveals increasing dementia prevalence in northern Sweden (Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011), a national sample of France (Bertrand et al., Reference Bertrand, Tzourio and Alperovitch2013), Hisayama, Japan (Sekita et al., Reference Sekita2010), and the province of Alberta, Canada (Jacklin et al., Reference Jacklin, Walker and Shawande2013). Notably, the number of prevalent dementia cases is forecast to increase to a greater extent in low and middle (227–264%) versus high income countries (116%) by 2050 (ADI, 2015).
To the best of our knowledge, two other original studies that examined simultaneous trends in dementia incidence and prevalence have been published within the last 10 years (Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013; Ng et al., Reference Ng2015). In the first, a prospective cohort study of two 6-year cohorts aged 75 and older from 1987–1989 and 2001–2004 in central Stockholm, Sweden, Qiu et al. (Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013) found that age-standardized dementia prevalence remained stable. Dementia incidence was not assessed directly; however, survival time based on 6-year follow-up was significantly longer for the later than earlier cohort, leading Qiu and colleagues to suggest that incidence decreased over the study period. In the second, a report based on a retrospective administrative health data study of the population aged 40 and older in Ontario, Canada, Ng et al. (Reference Ng2015) concluded that age-and sex-adjusted dementia prevalence increased over a 7-year period between 2004/2005 and 2010/2011, from 16.3 to 19.7 per 1,000 persons, and age- and sex-adjusted incidence decreased from 5.1 to 5.0 per 1,000 persons.
The value of using administrative health data to examine temporal trends in dementia incidence and prevalence can be illustrated in three key ways. The first of these is the investigation of possible impacts of population-level trends in modifiable risk factors throughout the lifecourse (early, midlife, and late life), on the incidence and prevalence of dementia (ADI, 2014). Currently, moderate to robust evidence exists for four domains of modifiable dementia risk factors: developmental (e.g. occupational status, education), psychological (e.g. depression, anxiety, sleep disorders), lifestyle or behavior (e.g. cigarette use), and cardiovascular (e.g. obesity, cholesterol, hypertension, diabetes) (ADI, 2014). Downward trends in dementia incidence over time in populations with documented improvements in these risk factors (e.g. improved education levels and reduced hypertension) would provide further evidence of the association between dementia and these risk factors. The second use of administrative health data in secular trend studies is to provide evidence for the association between trends in dementia and other population-level trends and interventions, including demographics (e.g. aging; Langa et al., Reference Langa2008; Sekita et al., Reference Sekita2010); life expectancy (Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012; Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013); treatment of chronic diseases (e.g. use of statins; Langa et al., Reference Langa2008; Hall et al., Reference Hall2009; and hypertensive medications; Langa et al., Reference Langa2008); treatment of cardiovascular diseases (Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011; Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012); health and social care for individuals with dementia (Sekita et al., Reference Sekita2010; Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011); and standard of living (Langa et al., Reference Langa2008). Third, current dementia projection methods are typically based on the assumption that certain factors will remain stable over time, such as age-specific dementia prevalence (ADI, 2015), mortality, and dementia risk factors (except demographics) (Rocca et al., Reference Rocca2011). Such projections do not adequately account for “changing patterns in risk factors” (Norton et al., Reference Norton, Matthews and Brayne2013), i.e. trends in population-level factors, that can be accounted for in studies based on administrative health data.
There have been several recent original Canadian studies concerning dementia prevalence, at the provincial level (Fransoo et al., Reference Fransoo, Martens, Burland, Prior and Burchill2009; Martens et al., Reference Martens2010; Gill et al., Reference Gill, Camacho, Poss, Bronskill, Camacho, Gruneir and Ho2011; Chartier et al., Reference Chartier2012; Jacklin et al., Reference Jacklin, Walker and Shawande2013). However, there have been few Canadian studies of trends in dementia prevalence (Jacklin et al., Reference Jacklin, Walker and Shawande2013; Ng et al., Reference Ng2015) and incidence (CSHA 2000; Tyas et al., Reference Tyas, Tate, Wooldrage, Manfreda and Strain2006; Ng et al., Reference Ng2015). Using linked administrative health data for the province of Saskatchewan for the time period between 2005/2006 and 2012/2013, the purposes of this study were to: (1) examine simultaneous age- and sex-specific temporal trends in dementia incidence and prevalence among individuals aged 45 and older, and (2) stratify any changes in incidence over time by database of identification.
Methods
Setting
The province of Saskatchewan is the middle of three Canadian prairie provinces and covers 651,000 km2 (Saskatchewan Bureau of Statistics, 2015). Between 2006 and 2013, the province's population grew 116,021 (11.7%) from 992,302 to 1,108,303 (Statistics Canada, 2014a). The proportion of the population aged 45–64 grew from 25.1% to 26.1% while the proportion aged 65 and older declined from 15% to 14.4%. The province's population growth of 74,047 between 2006 and 2011 (Saskatchewan Bureau of Statistics, 2014) was largely attributable to interprovincial migration (12,000; 16.2%) and immigration (28,000; 37.8%), with three times more immigrants during this period compared to 2001–2006 (9,800) (Statistics Canada, 2012). Among the 13 provinces and territories, Saskatchewan's growth during 2006–2011 was third largest at 6.7%, and larger than the national average at 5.9% (Statistics Canada, 2012).
Nearly all (99%) Saskatchewan residents receive provincial healthcare coverage (Downey et al., Reference Downey, Stang, Beck, Osei, Nichol and Strom2005) and constitute the “covered population” for the present study. Federally insured residents (federal prison inmates, members of the Canadian Forces, and Royal Canadian Mounted Police) are not included in the covered population (Saskatchewan Ministry of Health, 2012); however, their information is captured in hospital data. The Registered Indian population is not covered by the province's Prescription Drug Plan (Saskatchewan Ministry of Health, 2010) and therefore are not included in the Prescription Drug Database employed in the current study. Approximately 13% of the Saskatchewan population in 2012 were classified as Registered Indians (Aboriginal Affairs and Northern Development Canada, 2013).
Data sources
Data were extracted from seven provincial administrative health databases linked by a unique anonymized personal health services number (Saskatchewan Ministry of Health, 2010). Databases describing the demographic characteristics and insurance coverage for the population of Saskatchewan included the Person Health Registration System, Saskatchewan Resident Geography Database, and the Vital Statistics database. The databases from which the cohort were identified were the Hospital Discharge Abstract Database, Physician Services Claims Database, Prescription Drug Database, and the Resident Assessment Instrument – Minimum Data Set (RAI-MDS), i.e. Long-term Care Database.
From 2002 onwards, the Hospital Discharge Abstract Database includes 5-digit ICD-10-CA codes to record up to 25 diagnoses per record. The Physician Services Claims Database includes information used by physicians to claim payment from the provincial government for services provided to patients and a 3-digit ICD-9 diagnosis code associated with the service (maximum of one diagnosis code per service claim) (Saskatchewan Ministry of Health, 2010). The Prescription Drug Database includes information about drugs dispensed such as classification of the drug and drug identification number (DIN), with only Saskatchewan Formulary drugs eligible for coverage. The Long-term Care Database contains assessment information collected at admission to a residential care facility, at regular three-month intervals, and upon significant changes in clinical status (Morris et al., Reference Morris2010). Admission and quarterly assessment data were included in the present study.
Cohort
The case definition algorithm in the present study was developed over a three-stage process. Further details regarding the algorithm used in the current study are available elsewhere (Kosteniuk et al., Reference Kosteniuk2015).
Individuals aged 45 years or older at their first-ever recorded identification of dementia between April 1, 2005 and March 31, 2013 constituted the cohort. “Young onset dementia” (i.e. before age of 65) is estimated to affect approximately 6–9% of all prevalent cases (WHO, 2012) yet the true prevalence is unknown because epidemiological studies of dementia generally exclude those younger than 65 years (Lambert et al., Reference Lambert2014). Given the distinct needs and experiences of individuals with young onset dementia and their families and the deficiency of research in this area (Ducharme et al., Reference Ducharme, Kergoat, Antoine, Pasquier and Coulombe2014), as well as the need for a complete epidemiological picture, we chose to employ an age cut-off of 45 in the present study.
A “washout” period of 5 years prior to the first identification of dementia was used to ensure that we correctly identified incident dementia. Individuals entered the cohort either on their index date or April 1, 2005, whichever was later. They remained in the cohort until the earliest occurrence of any of the following: death, loss of their insurance (i.e. gap in insurance coverage of more than 3 days), or March 31, 2013. Individuals with a gap in their insurance of more than 3 days were not re-entered into the cohort.
Individuals were identified as a dementia case if they met at least one of the following criteria: >1 physician visit (ICD-9 codes 290, 294, 331, 797); >1 hospitalization (ICD-10-CA codes F00, F01, F02, F03, F04, F05.1, F06.8, F06.9, F09, F10.6, F10.7, F18.6, F18.7, F19.6, F19.7, G30, G31.0, G31.1, G91, R54); >1 prescription for a cholinesterase inhibitor (Aricept DINs 02232043, 02232044; Exelon DINs: 02242115–02242118, 02245240; Reminyl DINs: 02244298–02244300, 02266717, 02266725, 02266733); or – in the Long-term Care Database – a Cognitive Performance Scale (CPS) score of 2 or over and/or a disease category of Alzheimer's disease or dementia other than Alzheimer's disease.
Physician and hospital data are commonly used in administrative health data studies of dementia epidemiology, requiring at minimum one physician visit or hospitalization to identify a dementia case (Fransoo et al., Reference Fransoo, Martens, Burland, Prior and Burchill2009; Martens et al., Reference Martens2010; Gill et al., Reference Gill, Camacho, Poss, Bronskill, Camacho, Gruneir and Ho2011; Chartier et al., Reference Chartier2012; Manitoba Centre for Health Policy, 2012; Jacklin et al., Reference Jacklin, Walker and Shawande2013). Alzheimer's disease does not have a diagnostic test for confirmation purposes (St Germaine-Smith et al., Reference Germaine-Smith2012) and underdiagnosis of dementia is a significant problem (Boustani et al., Reference Boustani, Peterson, Hanson, Harris and Lohr2003; ADI, 2011; Connolly et al., Reference Connolly, Gaehl, Martin, Morris and Purandare2011). Therefore, the case definition for the present study prioritized sensitivity over specificity by including prescription drug and long-term care data to account for dementia cases that may not have been identified in physician or hospital data. Other medications may be used to treat Alzheimer's disease (e.g. memantine); however, the three medications included in the present study (Aricept, Exelon, and Reminyl) are the only cholinesterase inhibitors prescribed in Canada (Lee et al., Reference Lee, Hsiung, Seitz, Gill and Rochon2011) and are the most commonly used treatment of Alzheimer's disease in the country (Hogan, Reference Hogan2014). Moreover, cholinesterase inhibitors are typically not used for the treatment of other conditions and have been shown to have limited value in the treatment of attention-deficit hyperactivity disorder in a recent review (Bidwell et al., Reference Bidwell, McClernon and Kollins2011).
In the Long-term Care Database, the CPS consists of five measures in total: one of comatose status, two cognition (short term memory and cognitive skills for daily decision making), one communication, and one measure of activities of daily living (Morris et al., Reference Morris1994). The CPS categorizes individuals into one of seven levels of cognitive performance based on a score of 0 to 6 (Intact = 0; 1 = Borderline intact; 2 = Mild impairment; 3 = Moderate impairment; 4 = Moderate severe impairment; 5 = Severe impairment; 6 = Very severe impairment). A CPS score of 2 or higher is equivalent to an average Mini-Mental State Examination score of 19 or lower (Bartfay et al., Reference Bartfay, Bartfay and Gorey2013). This cut-off indicates dementia at the moderate to severe stage (Perneczky et al., Reference Perneczky, Wagenpfeil, Komossa, Grimmer, Diehl and Kurz2006) and possible mild to very severe impairment (Morris et al., Reference Morris1994). A CPS score of 2 or higher has been validated against physician diagnosis and found to be 68% sensitive and 92% specific in detecting dementia (Travers et al., Reference Travers, Byrne, Pachana, Klein and Gray2013), and against the Cambridge Examination for Mental Disorders of the Elderly-Revised (CAMDEX-R) and found to be 81% sensitive and 80% specific in detecting cognitive impairment (Paquay et al., Reference Paquay, De Lepeleire, Schoenmakers, Ylieff, Fontaine and Buntinx2007).
Independent variables
Age, sex, and administrative health database of first identification were the three independent variables included in the analysis. Age was represented by the categories of 45–54, 55–64, 65–74, 75–84, and 85 years and older. The four administrative health datasets included hospital, physician, prescription drug, and long-term care.
Statistical analysis
The age structure of the total cohort was used to adjust the sex-specific incidence rates and prevalence for age, and 95% confidence intervals (CI) were calculated for all crude and age-standardized rates.
Incident cases were identified for each 12-month period between April 1, 2005 and March 31, 2013. Incident cases met the case definition criteria and had not been previously identified during the washout period between April 1, 2000 and March 31, 2005. The numerator for each 12-month incidence rate was the number of people alive on April 1 of each year, who also met the case definition of dementia between April 1 of that year and March 31 of the following year. The denominator was the population at risk of developing incident dementia (i.e. after removing individuals with prevalent dementia for the same period, the remaining were aged 45 years or older on April 1 of each year with at least one day of health insurance coverage for the 12-month period).
Prevalent cases met the case definition criteria for each 12-month period from April 1 to March 31 for the years 2005 to 2013. The numerator for each 12-month prevalence was the number of people alive on April 1 of each year who met the case definition criteria at any time prior to April 1 of that year. Those individuals at risk for prevalent dementia (i.e. all individuals in the covered population aged 45 years or older on April 1 of each year with at least one day of health insurance coverage for the 12-month period) constituted the denominator.
For incidence and prevalence, we calculated the percentage changes between 2005/2006 and 2012/2013 in absolute number (n), percentage, population, and age-standardized rate per 1,000, by dividing the difference between the two figures by the earlier figure and multiplying by 100. Percentage changes in age-standardized incidence rates and prevalence per 1,000 were compared for significant differences (p < 0.05) using the χ2 test, and 95% CI were calculated for all crude and age-standardized rates. All analyses were completed with SAS 9.3 (SAS Institute Inc, Cary NC).
Ethical considerations
The University of Saskatchewan Biomedical Research Ethics Board granted ethics approval for this study (Bio-REB #12-339).
Results
Incidence
As shown in Figure 1, the overall age-standardized incidence rate of dementia among individuals 45 years and older declined gradually and steadily from 2005/2006 until 2010/2011, rising slightly in 2011/2012 before dropping again in 2012/2013. Table 1 indicates that the annual population rose steadily each year between 2005/2006 to 2012/2013. As shown in Table 2, the population increased by 11.38% from 403,123 to 449,012 while the absolute number of overall incident cases dropped by 3.51% from 3,389 to 3,270 between 2005/2006 and 2012/2013. The overall age-standardized incidence rate declined significantly by 11.07% (p < 0.0001) from 8.41 to 7.48 per 1,000 over the 8-year period.
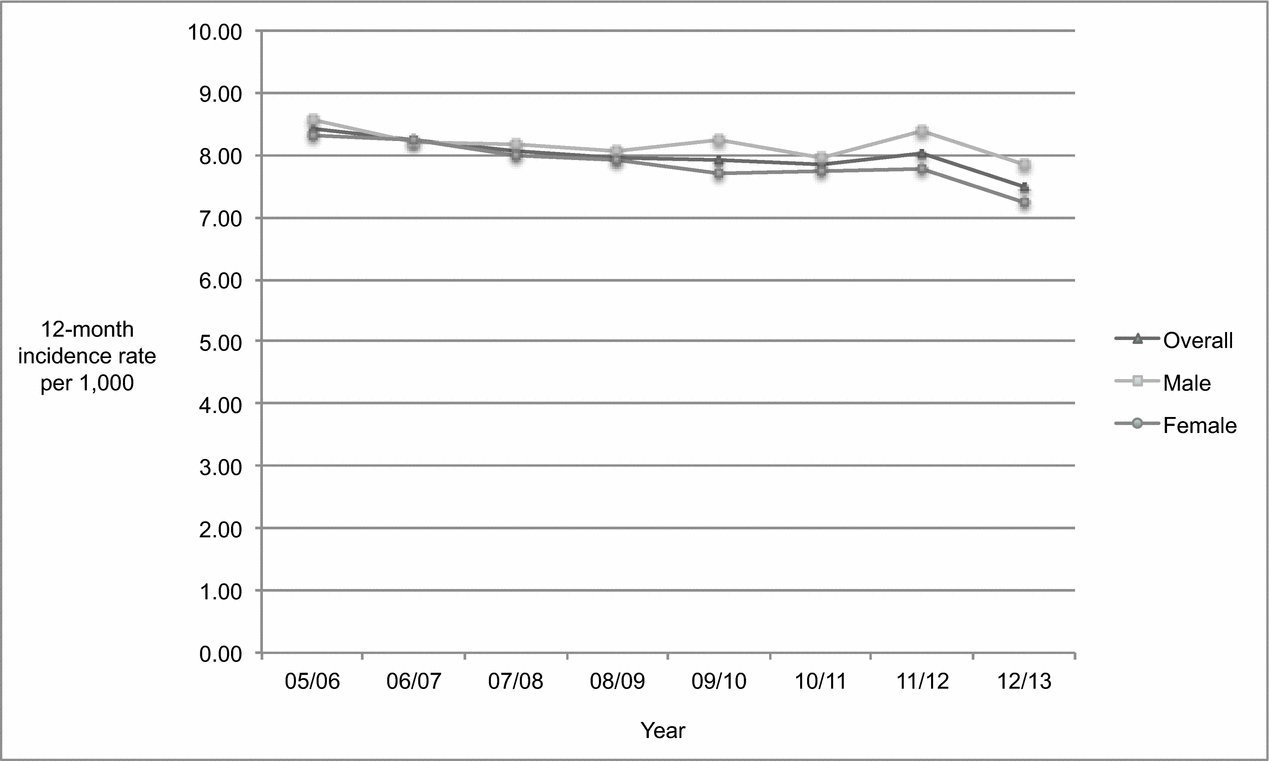
Figure 1. Age-standardized 12-month incidence rate of dementia among adults 45 years of age and older, Saskatchewan, from 2005/2006 to 2012/2013.
Table 1. Twelve-month incidence and prevalence of dementia among adults 45 years of age and older, Saskatchewan, from 2005/2006 to 2012/2013
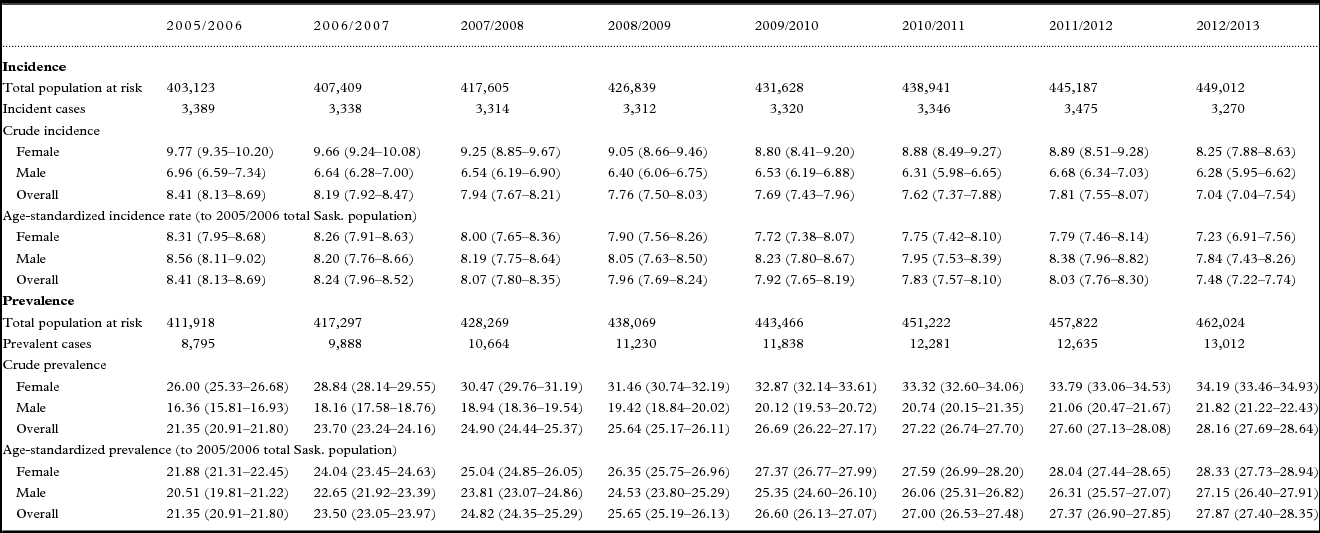
Table 2. Change in 12-month incidence of dementia among adults 45 years of age and older, Saskatchewan, 2005/2006 to 2012/2013
a Test of difference between age-standardized dementia incidence rate in 2005/2006 versus 2012/2013.
b Change in crude rate per 1,000.
Table 2 shows that although the female and male populations increased between 2005/2006 and 2012/2013 (10.12% and 12.73% respectively), the absolute number of incident cases among females dropped while the absolute number of incident cases among males rose. Consequently, the age-standardized incidence rate decreased more markedly among females than males, dropping significantly by 12.97% (p < 0.0001) among females (from 8.31 to 7.23 per 1,000) compared to 8.39% (p = 0.0072) among males (from 8.56 to 7.84 per 1,000). The proportion of incident cases attributed to females versus males dropped as well, by 3.66% from 59.89% to 57.71%. The age-standardized incidence rate was slightly higher among males than females in 2005/2006 (8.56 vs. 8.31 per 1,000) and remained so in 2012/2013 (7.84 vs. 7.23 per 1,000).
Overall mean age at identification in 2005/2006 (81.67 ± 9.98 years) did not change significantly (p = 0.24) in 2012/2013 (81.97 ± 10.70 years). As shown in Table 2, the population changed most substantially in the 55–64 and 65–74 age groups, increasing 16–31% among females and 20–32% among males. Despite this, the age-standardized incidence rate in the 55–64 age group did not change significantly over time for either sex. Among females, significant declines in age-standardized incidence rates were apparent in the three oldest age groups, ranging from 11.97% (p = 0.0377) in those aged 85 and older [from 74.53 to 65.61 per 1,000 to 15.40% (p = 0.0396) in those aged 65–74 (from 4.85 to 4.10 per 1,000)]. A significant decline of 18.97% (p = 0.0136) in the age-standardized incidence rate among males was apparent only among those aged 65–74 (from 5.25 to 4.25 per 1,000). The population remained stable and neither sex in the 45–54 age group experienced significant changes in age-standardized incidence rates over time.
In terms of the databases where incident cases of dementia were first identified, the greatest proportion were first identified in Long-term Care in 2005/2006 (35.35%) and 2012/2013 (34.98%) (Table 2). The declines over time in the crude incidence rates per 1,000 were significant across every database with the exception of Prescription Drug, with similar declines in the Physician (14.17%; p = 0.0007), Long-term Care (14.14%; p = 0.0002) and Hospital databases (12.97%; p = 0.0022).
Prevalence
Figure 2 shows that the overall age-standardized prevalence of dementia among those aged 45 and older increased between 2005/2006 to 2012/2013. Most of the increase took place in the first four years of the study period, with the upward trend slowing between 2009/2010 and 2012/2013. Over the 8-year period, the absolute number of overall prevalent cases rose 47.95% from 8,795 to 13,012, compared to an increase of 12.16% in the population from 411,918 to 462,024 (Tables 1 and 3). The overall age-standardized prevalence increased significantly (p < 0.0001) by 30.54% over time from 21.35 to 27.87 per 1,000.
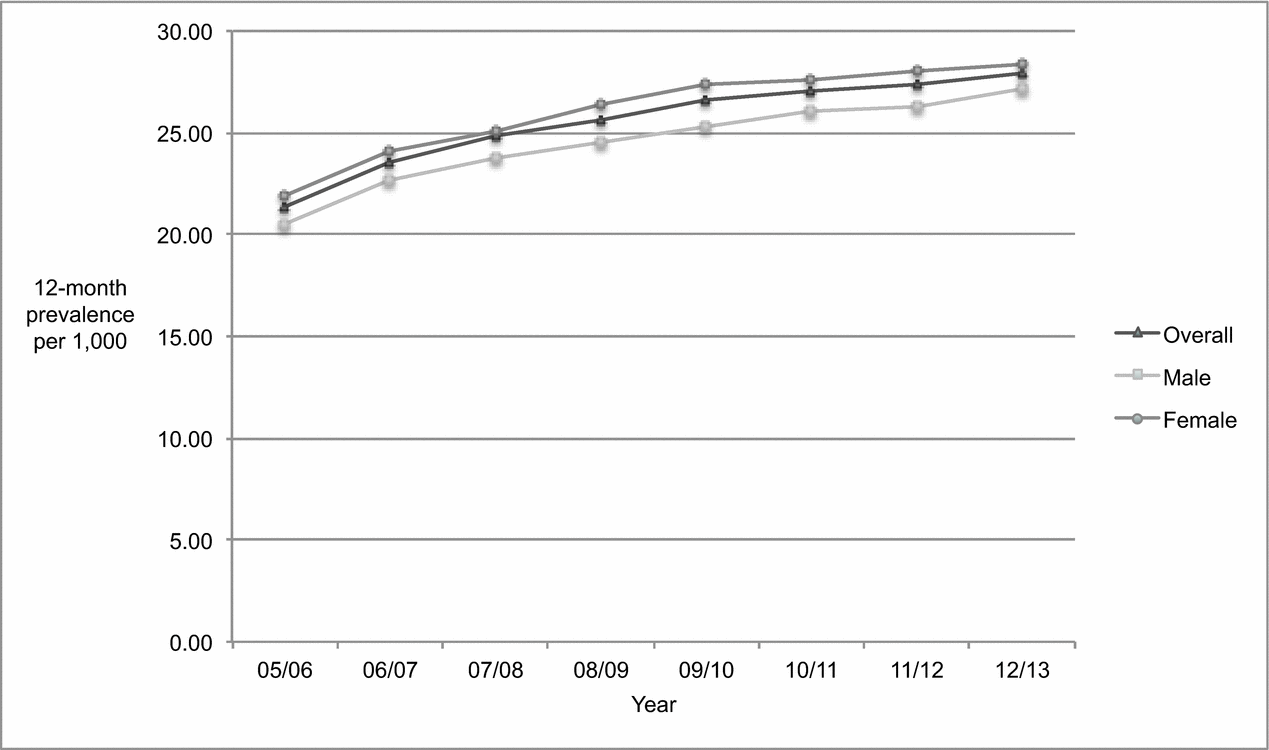
Figure 2. Age-standardized 12-month prevalence of dementia among adults 45 years of age and older, Saskatchewan, from 2005/2006 to 2012/2013.
Table 3. Change in 12-month prevalence of dementia among adults 45 years of age and older, Saskatchewan, 2005/2006 to 2012/2013

a Test of difference between age-standardized dementia prevalence in 2005/2006 versus 2012/2013.
As shown in Table 3, the population increased slightly more among males than females (13.36% vs. 11.05%), as did the absolute number of prevalent cases (51.22% vs. 46.03%). As a result, the age-standardized prevalence increased significantly (p < 0.0001) in both sexes, but to a slightly greater degree by 32.38% among males (from 20.51 to 27.15 per 1,000) compared to 29.48% among females (from 21.88 to 28.33 per 1,000). The proportion of prevalent cases attributed to males relative to females rose as well, from 36.94% to 37.76% (2.33%). However, the age-standardized incidence rate was slightly higher among females than males in 2005/2006 (21.88 vs. 20.51 per 1,000) and remained so in 2012/2013 (28.33 vs. 27.15 per 1,000).
Similar to increases in the population at risk for incident cases, the largest increases in the population at risk for prevalent cases took place in the 55–64 and 65–74 age groups. With the exception of the 45–54 age group, significant increases in age-standardized prevalence were apparent in every age group for both sexes. The largest increase in the age-standardized prevalence for both sexes took place in the 55–64 age group (107.08% female, p < 0.0001; 48.72% male, p < 0.0001) and the smallest increase was experienced by the 85 and older age group (23.98% female, p < 0.0001; 23.86% male; p < 0.0001).
Discussion
Using a population-based retrospective cohort design, we identified incident and prevalent cases of dementia between April 1, 2005 and March 31, 2013 in linked administrative health databases (Hospital Discharge Abstracts, Physician Service Claims, Prescription Drug, and RAI- MDS, i.e. Long-term Care), among individuals 45 years and older at first identification of dementia.
Considering the first study objective to investigate simultaneous age- and sex-specific temporal trends in dementia incidence and prevalence, we found the overall age-standardized incidence rate declined significantly by 11.07% and the age-standardized prevalence increased significantly by 30.54% over the 8-year study period. Overall, the incidence rate declined from 8.41 to 7.48 per 1,000 despite an 11.38% increase in the overall population. Although both sexes experienced significant declines in the incidence rate over time, females experienced a slightly larger decrease than males (12.97% vs. 8.39%). The age-standardized incidence rate remained higher among males than females in 2012/2013 (7.84 vs. 7.23 per 1,000) as in 2005/2006 (8.56 vs. 8.31 per 1,000). Among females, significant decreases occurred only in the three oldest age groups, with the largest decline in the 65–74 age group. Among males, only the 65–74 age group experienced a significant decline over the 8-year period.
Overall, the age-standardized prevalence of dementia increased significantly by 30.54% from 21.35 to 27.87 per 1,000, and the population increased by 12.16% between 2005/2006 and 2012/2013. Males experienced a slightly larger increase than females in the age-standardized prevalence over time (32.38% vs. 29.48%). The age-standardized prevalence was higher among females than males in 2005/2006 (21.88 vs. 20.51 per 1,000) and remained so in 2012/2013 (28.33 vs. 27.15 per 1,000). Significant increases were apparent in every age group for both sexes (except those 45–54), with the largest increment in the 55–64 age group and the smallest increment in the 85 and older age group for both sexes.
Considering the second study objective to stratify the changes in incidence over the 8-year study period by database of identification, significant decreases in the crude incidence rate per 1,000 were apparent in 3 of the 4 databases examined, with declines of 13–14% across Hospital Discharge Abstracts, Physician Service Claims, and RAI-MDS (i.e. Long-term Care).
Incidence
Our finding of declining dementia incidence over time is consistent with four original studies published within the last 10 years on the topic of incidence trends. Two were separate field studies (i.e. two-phase studies with screening followed by a structured clinical evaluation) in Rotterdam, the Netherlands (Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012) and Stockholm, Sweden (Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013); the third study was based on medical records in Rochester, US (Rocca et al., Reference Rocca2011) and the most recent study was based on administrative health data from the province of Ontario, Canada (Ng et al., Reference Ng2015). Compared to a 1.4% per year decline in the current study, incidence rates declined an average of 2.5–3% per year in two of the four studies (Rocca et al., Reference Rocca2011; Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012), approximately 4.2% over a 7-year period in a third study (Ng et al., Reference Ng2015), and an unspecified amount in a fourth study (Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013). Similar to the present study, Schrijvers et al. (Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012) observed a slightly greater decrease in the incidence rate over time in females than males; however, in contrast to the present study, the incidence rate was higher among females than males at both time points.
Prevalence
The results of five original studies were in line with our finding of rising dementia prevalence over time, including separate field studies in Hisayama, Japan (Sekita et al., Reference Sekita2010) and northern Sweden (Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011) and administrative data studies in France (Bertrand et al., Reference Bertrand, Tzourio and Alperovitch2013) and the Canadian provinces of Alberta (Jacklin et al., Reference Jacklin, Walker and Shawande2013) and Ontario (Ng et al., Reference Ng2015). At 3.82% per year, the average annual growth in prevalence in the present study is in the lower range compared to other studies, which varied between 1.9–9.8% (Sekita et al., Reference Sekita2010; Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011; Bertrand et al., Reference Bertrand, Tzourio and Alperovitch2013; Jacklin et al., Reference Jacklin, Walker and Shawande2013; Ng et al., Reference Ng2015). In the present study, males experienced a slightly larger increase than females in prevalence over time, whereas Sekita et al. (Reference Sekita2010) observed the reverse. However, prevalence remained higher in females than males over time in the present study, in line with findings from two studies that observed increasing prevalence trends (Sekita et al., Reference Sekita2010; Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011).
Contrary to the results from the present study, three original studies reported a stable temporal trend in dementia prevalence, including an administrative health data study in Germany (Doblhammer et al., Reference Doblhammer, Fink and Fritze2015) and separate field studies in Indianapolis, US (Hall et al., Reference Hall2009) and Stockholm, Sweden (Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013). An additional three original studies reported a downward temporal trend, namely separate field studies in a national US sample (Langa et al., Reference Langa2008), Zaragoza, Spain (Lobo et al., Reference Lobo2007), and regions of England and Wales (Matthews et al., Reference Matthews2013).
Possible explanations
Recently published reviews and commentaries offer several possible explanations for decreasing dementia incidence and prevalence over time, as well as for increasing prevalence (Larson and Langa Reference Larson and Langa2012; Banerjee Reference Banerjee2013; Larson et al., Reference Larson, Yaffe and Langa2013; Whalley and Smyth Reference Whalley and Smyth2013; ADI, 2015; Lee Reference Lee2014; Sachev Reference Sachev2014; Wu et al., Reference Wu2015). Preliminary supporting evidence for these observations is provided by findings from several original studies, wherein some of these explanations were tested directly (Langa et al., Reference Langa2008; Hall et al., Reference Hall2009; Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012; Elwood et al., Reference Elwood2013; Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011), and others wherein speculations were made on the basis of population-level trends and interventions in modifiable risk factors and other factors (e.g. demographics) (Lobo et al., Reference Lobo2007; Langa et al., Reference Langa2008; Sekita et al., Reference Sekita2010; Rocca et al., Reference Rocca2011; Matthews et al., Reference Matthews2013; Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013). First, cognitive reserve as an outcome of higher education and occupational complexity has been cited as a protective factor (Langa et al., Reference Langa2008) and rising education levels and intellectual demands over time have been linked to declining incidence and prevalence of dementia in later cohorts (Langa et al., Reference Langa2008; Hall et al., Reference Hall2009; Rocca et al., Reference Rocca2011; Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012; Matthews et al., Reference Matthews2013). In terms of the present study, education levels have been rising in Saskatchewan, reflected in an annual 2.8% growth in the proportion of post-secondary graduates aged 25–64 between 2000 and 2012 (Statistics Canada, 2013a).
Recent evidence from a 25-year longitudinal study supports an association between reduced risk of dementia and healthy lifestyle or behavior (e.g. non-smoking, physical activity, healthy diet, and limited alcohol intake) (Elwood et al., Reference Elwood2013). Increased uptake of healthy behaviors over time has been linked to declining dementia trends (Lobo et al., Reference Lobo2007; Hall et al., Reference Hall2009; Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013) as have reduced cardiovascular risks such as prevention of heart disease (Matthews et al., Reference Matthews2013), and decreased hypertension (Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013), cholesterol (Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013), and stroke (Rocca et al., Reference Rocca2011). However, a trend of increasing dementia prevalence in Japan has also been attributed to rising rates of obesity, hypercholesterolemia, and other metabolic disorders (Sekita et al., Reference Sekita2010). Population data indicate that while the rate of non-smoking, physical activity, and fruit/vegetable consumption increased in Saskatchewan over the study period, so too did the rates of obesity, diabetes, and high blood pressure (Statistics Canada, 2013b; Elliot, Reference Elliott2014).
Recent studies support an association between temporal trends of dementia decline and improved treatment of vascular risks (Lobo et al., Reference Lobo2007; Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013) such as the use of antithrombotic and lipid-lowering drugs (Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012), antihypertensive medications (Langa et al., Reference Langa2008; Hall et al., Reference Hall2009) and statins (Langa et al., Reference Langa2008; Hall et al., Reference Hall2009; Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012). The most recent available population-level data for the study period indicate declining annual rates of mortality in Saskatchewan due to major cardiovascular diseases (Statistics Canada, 2014b), heart diseases, and cerebrovascular diseases (2003–2009) (Statistics Canada, 2013b).
Furthermore, increased dementia prevalence reflects lengthier duration of survival with dementia, possibly owing to improved care and treatment, such as better health services and institutional care (Sekita et al., Reference Sekita2010) and increased cholinesterase inhibitors prescriptions (Mathillas et al., Reference Mathillas, Lovheim and Gustafson2011). Langa et al. (Reference Langa2008) proposed the “compression of cognitive morbidity” hypothesis that declining dementia trends demonstrate a delay of dementia to older age, reflecting the positive association over time between quality of life and brain health. Mathillas et al. (Reference Mathillas, Lovheim and Gustafson2011) suggested that better treatment of cardiovascular risks and reduced mortality due to cardiovascular disease contributed to a growing pool of Swedish older adults aged 85 and older at risk of dementia, thereby reflecting a trend of increasing dementia prevalence in this age cohort.
In terms of the present study, immigration accounted for 37.8% of total population growth in Saskatchewan between 2006 and 2011 (Statistics Canada, 2012). It is plausible that our observation of declining dementia incidence despite population growth was partly due to a limited recognition of dementia during encounters between healthcare professionals and older adult immigrants to Saskatchewan. It is also plausible that health selective migration, whereby older adult immigrants have better than average health (Norman et al., Reference Norman, Boyle and Rees2005), was partly responsible for this decline.
Variations in the direction and magnitude of change over time in incidence and prevalence across studies may be partly due to differences in diagnostic and classification criteria (Wu et al., Reference Wu, Matthews and Brayne2014) and sample or population characteristics (e.g. age cut-offs, demographic trends in populations). For example, given the higher prevalence among institutionalized compared to community-dwelling populations (Hoffman et al., Reference Hoffman, Kaduszkiewicz, Glaeske, van den Bussche and Koller2014), excluding nursing home residents in field studies (e.g. Langa et al., Reference Langa2008; Hall et al., Reference Hall2009) possibly underestimates dementia prevalence overall. Methodological approaches (e.g. observation periods) may also contribute to variations. For instance, in comparison to field studies, administrative health data studies such as the present study tend to underestimate the true number of individuals with dementia because dementia tends to be under-recognized in the healthcare system (Lambert et al., Reference Lambert2014). Moreover, evidence in some high income nations of declining incidence trends (Rocca et al., Reference Rocca2011; Schrijvers et al., Reference Schrijvers, Verhaaren, Koudstaal, Hofman, Ikram and Breteler2012; Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013) and stable or downward prevalence trends (Lobo et al., Reference Lobo2007; Langa et al., Reference Langa2008; Hall et al., Reference Hall2009; Matthews et al., Reference Matthews2013; Qiu et al., Reference Qiu, Strauss, Backman, Winblad and Fratiglioni2013; Doblhammer et al., Reference Doblhammer, Fink and Fritze2015), may reflect a positive association between national wealth, public health, and healthcare and therefore hinder generalization of findings to low and middle income countries where population aging and cardiovascular risk factors tend to be on the rise (ADI, 2015; Wu et al., Reference Wu2015).
Several interrelated factors potentially account for the limited impact of the declining dementia incidence rate on the prevalence of dementia in the current study. The primary explanation may be that the 8-year observation period was too brief to demonstrate an impact. Second, rising prevalence despite declining incidence in the present study indicates that survival time with dementia was also increasing, from 2.56 years in 2005/2006 (21.53/8.41 in 2005/2006) to 3.73 years in 2012/2013 (27.87/7.48). Increased survival time and prevalence may be due to identification of dementia in earlier stages (ADI, 2015) and improved treatment after identification. Lastly, the declining provincial mortality rate and growth of the overall population aged 45 and older minimized the impact of declining incidence upon prevalence during the short 8-year observation period. Beginning in 2009/2010, declining incidence may have begun to manifest in a relatively slower increase in prevalence compared to pre-2009/2010, perhaps signaling the beginning of a stabilizing trend in dementia prevalence.
Limitations
Administrative health data is collected for purposes other than disease surveillance, and as such, several limitations are associated with the use of administrative health data to determine incidence and prevalence of dementia. First, underdiagnosis of dementia is a significant issue, with studies showing that 31–69% of primary care patients with dementia do not receive a formal documented diagnosis (Boustani et al., Reference Boustani, Peterson, Hanson, Harris and Lohr2003; Bradford et al., Reference Bradford, Kunik, Schulz, Williams and Singh2009; van den Dungen et al., Reference van den Dungen2012). Moreover, physician services claims permit a maximum of one diagnosis code per claim, therefore diseases due to dementia may not be captured in these claims if patients present with other problems. As a result, studies based on administrative health data tend to produce underestimations of prevalence and incidence in comparison to field studies (Lambert et al., Reference Lambert2014). However, data linkage across sectors is possible in administrative health data studies, allowing community and institution-dwelling populations to be examined as a whole for a more complete picture of dementia epidemiology, in contrast to field studies which may not combine these populations (e.g. Langa et al., Reference Langa2008; Hall et al., Reference Hall2009). Second, all of the data sources in the present study included the Registered Indian population, with the exception of the prescription drug database. However, Registered Indians who were not identified as cases in the prescription drug database were likely identified in one of the other three administrative health databases. Therefore, their exclusion from the prescription drug database may have contributed to a minor underestimation of the increase in prevalence over the study period given the faster rise in dementia prevalence over time in First Nations compared to non-First Nations documented by Jacklin and colleagues (Reference Jacklin, Walker and Shawande2013). Third, individuals excluded from the cohort due to interrupted health insurance coverage (i.e. a gap in insurance coverage of more than 3 days) accounted for 4.2% of the total cohort over the study period. Compared to individuals without gaps, those with gaps were more likely to be male (40.4% with no gap vs. 45.1% with gap) and Registered Indian (2.1% with no gap vs. 9.7% with gap). Registered Indians comprised only 2.4% of the dementia cohort and therefore the overall estimates of incidence and prevalence reported in the present study were not likely affected. However, despite the small proportion of people excluded from the study, the sex-specific estimates of prevalence were likely to have been affected because of the additive effect of disproportionately excluding men. Specifically, each year the prevalence of dementia will be further underestimated in men compared to women because each year, just a few more men than women will be excluded. The difference in the prevalence of dementia between men and women will appear more pronounced with each passing year. Fourth, excluding a prescription of memantine from the case definition algorithm may have resulted in a slight underestimation of prevalence and incidence, in cases that had not been identified with a cholinesterase prescription or in one of the other three databases. Finally, our study period of 8 years may be too short to discern a consistent and reliable pattern or trend in dementia over time.
Conclusions
Despite some limitations, administrative health data is a valuable research tool for tracking trends in dementia incidence and prevalence. The present study demonstrated that over an 8-year period in the province of Saskatchewan, the age-standardized incidence rate of dementia declined among individuals aged 45 and older while the age-standardized prevalence of dementia simultaneously increased. These trends indicate that the average survival time with dementia was also increasing, suggesting the possibilities that recognition of dementia is taking place in earlier stages and treatment is improving. As individuals live longer with dementia, similar to other chronic diseases, they require active care and monitoring for an extended period of time (Bergman, Reference Bergman2009; ADI, 2014). To spur improvements in dementia care and address increasing cost burdens, several G7 nations have developed national dementia strategies (France, Japan, United Kingdom, United States, Italy). Canada currently does not have a national dementia plan, despite an estimated 500,000 Canadians living with dementia in 2008 and over 100,000 incident cases developing each year (Dudgeon, Reference Dudgeon2010). Further reduction in dementia incidence is certainly possible with the type of concentrated focus that a national strategy promises, and future research should track these developments.
Conflict of interest
None.
Description of authors’ roles
J. Kosteniuk, D. Morgan, J. Quail, and G. Teare conceived of the study. J. Quail and G. Teare acquired the data and J. Quail performed the analyses. J. Kosteniuk, D. Morgan, J. Quail, M. O'Connell, A. Kirk, and M. Crossley constructed the algorithm for case identification. J. Kosteniuk, D. Morgan, J. Quail, M. O'Connell, A. Kirk, M. Crossley, G. Teare, N. Stewart, V. Dal Bello-Haas, L. McBain. H. Mou, D. Forbes, and A. Innes undertook the study design and data interpretation. J. Kosteniuk wrote the initial draft of the manuscript. All authors critically revised the manuscript, read, and approved the final manuscript.
Disclaimer
This study is based in part on de-identified data provided by the Saskatchewan Ministry of Health through the Health Quality Council. The interpretation and conclusions contained herein do not necessarily represent those of the Government of Saskatchewan or the Saskatchewan Ministry of Health.
Acknowledgments
Study funding was provided by an Applied Chair in Health Services and Policy Research Award to DGM from the Saskatchewan Health Research Foundation (#2104) and Canadian Institutes of Health Research (#ACH 93185). JMQ and GFT are both employed by the Saskatchewan Health Quality Council which contributed, in-kind, portions of their time for this project.







