Article contents
Highly conductive ink made of silver nanopolyhedrons through an ecofriendly solution process
Published online by Cambridge University Press: 18 February 2011
Abstract
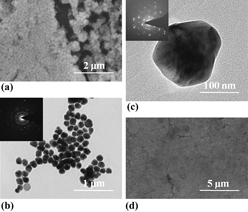
An ecofriendly process has been successfully developed to synthesize the polycrystalline silver nanopolyhedrons with a high yield at large scale. By using tannic acid in the presence of poly (vinyl pyrrolidone) (PVP), high quality silver nanopolyhedrons were obtained in an aqueous one-pot reaction without any templates or auxiliaries. The film made from the silver nanostructures exhibits an electrical conductivity higher than 104 S/cm on both rigid and flexible substrates. The supreme mechanical strength of this silver film recommends its wide application in printing and flexible electronics.
Keywords
- Type
- Materials Communications
- Information
- Copyright
- Copyright © Materials Research Society 2011
Footnotes
The purpose of this Materials Communications section is to provide accelerated publication of important new results in the fields regularly covered by Journal of Materials Research. Materials Communications cannot exceed four printed pages in length, including space allowed for title, figures, tables, references, and an abstract limited to about 100 words.
These authors contributed equally to this work.
References
REFERENCES
- 1
- Cited by


