Article contents
Influence of structure of activated carbon with superhigh specific surface area on hydrogen storage capacity
Published online by Cambridge University Press: 11 February 2013
Abstract
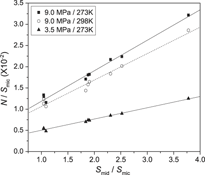
Activated carbon adsorbents with superhigh specific surface areas (SHACs), which are used as adsorbents, were prepared by chemical activation of petroleum coke with potassium hydroxide. We investigated the influence of specific surface area on hydrogen desorption capacity using SHACs with the same pore size distribution, whereas the effect of pore size distribution on hydrogen desorption capacity was studied using SHACs with same specific surface area. Results revealed that hydrogen desorption capacity (N) increased with higher specific surface area (S) of SHAC adsorbents, according to the linear relation: N = k·S + b (k > 0). At 273 K and 9.0 MPa, hydrogen desorption capacity of 20.96 mmol/g (4.02 wt%) was observed on a SHAC adsorbent with a specific surface area of 3348 m2/g. There was a linear relationship between hydrogen desorption capacity and mesopore percentage in SHAC adsorbents, described as: N = k2·Xmic + b (k2 > 0). Hydrogen desorption per unit mesopore surface amounted to 0.72 mmol/m2.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 8
- Cited by




