Article contents
Magnetic properties and thermal stability of polyvinylidene fluoride—Fe2O3 nanocomposites
Published online by Cambridge University Press: 03 January 2020
Abstract
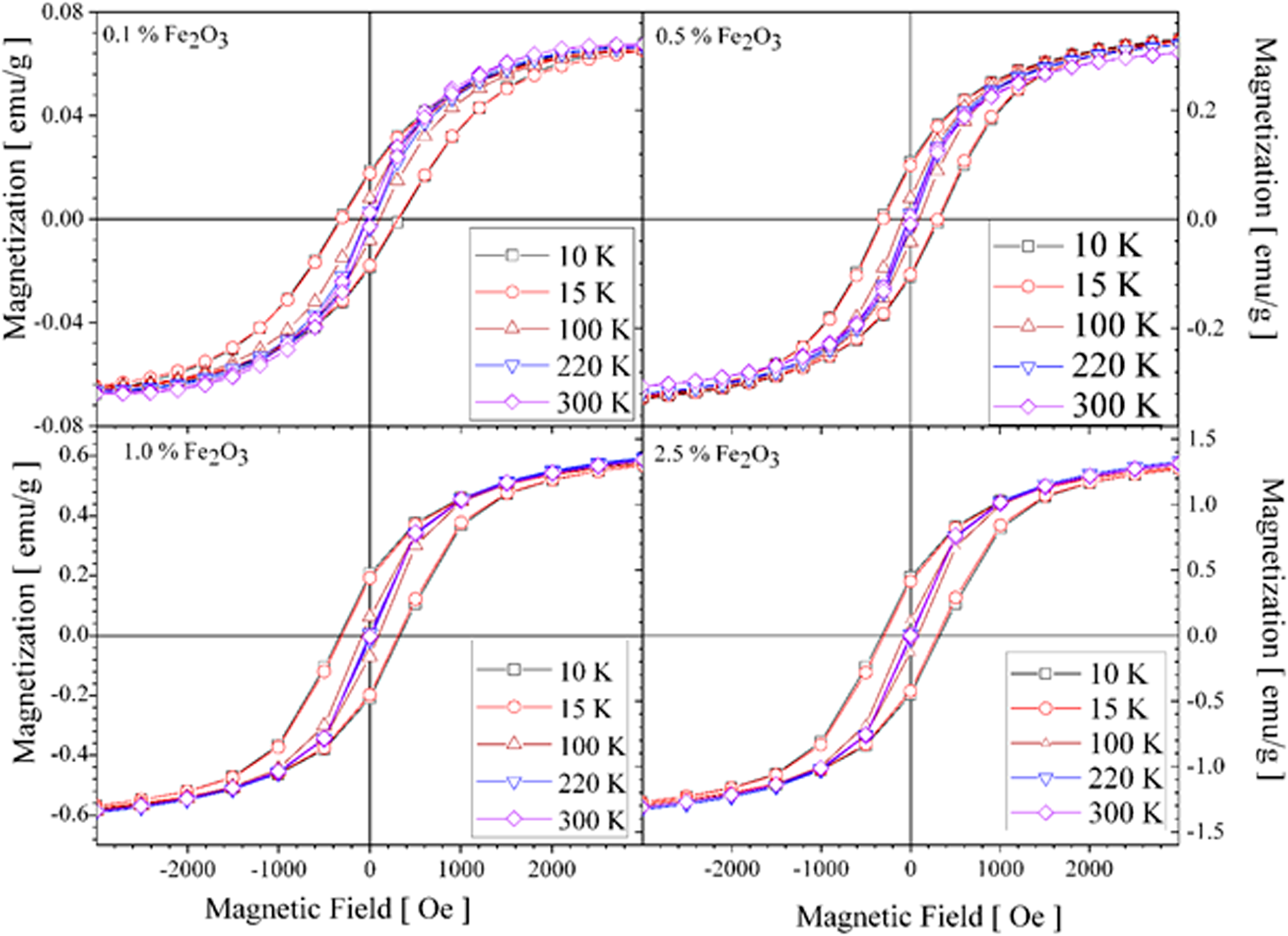
Nanocomposites of polyvinylidene fluoride loaded with various amounts of γ-Fe2O nanoparticles, with an average size ranging between 20 and 40 nm, have been obtained by melt mixing and investigated using various experimental techniques [Superconducting Quantum Interference Device, Mössbauer, and Thermogravimetric Analysis]. Magnetic and Mössbauer measurements confirmed the presence of maghemite and a trace of a paramagnetic iron compound. Magnetic data are consistent with a blocking temperature close to room temperature (RT), showing a decrease in the coercive field as the temperature is increased. A weak exchange bias was noticed in all nanocomposites investigated at all temperatures and tentatively ascribed to surface spin disorder. The temperature dependence of the coercive field obeys the Kneller law. The nanocomposites exhibit superparamagnetic behavior near RT. Most magnetic measurements have been performed below the blocking temperature, revealing thus a complex behavior. The dependence of the mass loss derivative versus temperature, as obtained by thermogravimetric analysis, exhibits a single peak due to the thermal degradation of the polymeric matrix. A weak increase in the thermal stability of the polymeric matrix upon loading with maghemite is reported.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 6
- Cited by





