Article contents
Preparation of chitosan/safflower and ligusticum wallichii polysaccharides hydrogel for potential application in drug delivery and tissue engineering
Published online by Cambridge University Press: 11 July 2017
Abstract
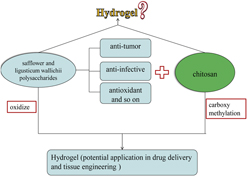
Biological hydrogel is important in drug delivery system and tissue engineering. In this paper, we prepared a series of biological hydrogels with N,O-carboxymethyl chitosan (CS) and oxidized safflower and ligusticum wallichii polysaccharide-II (oxidized SLWP-II). Morphological analysis indicated the N,O-carboxymethyl CS/oxidized SLWP-II hydrogels (CSLHs) had porous interior structures, pore diameter ranged from tens to hundreds of micrometers. In vitro release test showed, with proportion of N,O-carboxymethyl CS to oxidized SLWP increasing from 1:1 to 1:3, cumulative release of bovine serum albumin decreased from 99 to 82%. In vitro cytotoxicity study showed that the developed hydrogels were not cytotoxic during one week of culturing with WI-38 cells, and they have a role in promoting cell proliferation. So the N,O-carboxymethyl CS/oxidized safflower and ligusticum wallichii polysaccharide-II hydrogels might have potential application in the drug delivery system and tissue engineering.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Susmita Bose
References
REFERENCES
- 3
- Cited by





