Introduction
Cardichelyon rogerwoodi Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013 is an enigmatic, big-headed fossil turtle from the Paleogene of the western United States (Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013). Even though this taxon had been noted in the literature under various informal names since the mid 1970s (Estes, Reference Estes1975; Bartels, Reference Bartels1980, Reference Bartels1983; Hutchison, Reference Hutchison1980, Reference Hutchison, Prothero and Berggren1992, Reference Hutchison, Aubry, Lucas and Berggren1998; McCord, Reference McCord1996; Holroyd and Hutchison, Reference Holroyd and Hutchison2000; Holroyd et al., Reference Holroyd, Hutchison and Strait2001; Hutchison and Frye, Reference Hutchison and Frye2001), it was only formally named and described recently (Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013). In addition to rich, fragmentary material from the late Paleocene (Clarkforkian) to early Eocene (Wasatchian) of Wyoming, Cardichelyon rogerwoodi is known from more complete specimens, including complete shells and a partial skull, that document important anatomical details of systematic value. Estes (Reference Estes1975), Bartels (Reference Bartels1980, Reference Bartels1983), Hutchison (Reference Hutchison1980, Reference Hutchison, Prothero and Berggren1992, Reference Hutchison, Aubry, Lucas and Berggren1998), and McCord (Reference McCord1996) highlighted affinities with pond turtles (Emydidae and Geoemydidae), but did not discuss this assessment. In the type description, Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) referred Cardichelyon rogerwoodi to the Asian big-headed turtles (Platysternidae), but only listed the presence of multiple musk-duct foramina and a macrocephalic skull as character evidence. This hypothesis was tentatively confirmed by the phylogenetic analysis of Vlachos (Reference Vlachos2018). The possible presence of a fossil platysternid in the Paleogene of North America has biogeographic significance because the group has otherwise only been reported from the Tertiary of Asia (see Danilov et al., Reference Danilov, Syromyatnikova, Sukhanov, Lopatin and Zelenkov2018 for a recent summary).
We here report that the type material of Cardichelyon rogerwoodi exhibits characters that were not noted by previous authors and that suggest kinosternoid, rather than testudinoid affinities for this taxon. The purpose of this contribution is to re-describe the shell of this turtle, to discuss the available character evidence, and to provide an alternative phylogenetic assessment.
Materials and methods
The type material of Cardichelyon rogerwoodi was collected over the course of the 1930s and 1940s by teams from Princeton University and is now held at YPM. The descriptions provided herein are based on personal observations of this material. Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) described and figured additional material from other localities that is housed at UCMP. Because we did not observe this material in person, we regularly refer to these descriptions as a source of additional information, but cannot comment on their accuracy. The extant turtles utilized in the phylogenetic analyses or for comparisons are housed at AMNH, BMNH, FMNH, and USNM.
Repositories and institutional abbreviations
Herpetology, American Museum of Natural History (AMNH), New York, New York, USA; Herpetology, Natural History Museum (BMNH), London, United Kingdom; Herpetology, Field Museum of Natural History (FMNH), Chicago, Illinois, USA; University of California Museum of Paleontology (UCMP), Berkeley, California; USA; Herpetology, National Museum of Natural History (USNM), Washington DC, USA; Vertebrate Paleontology, Yale Peabody Museum of Natural History (YPM), New Haven, Connecticut, USA.
Systematic paleontology
Testudines Batsch, Reference Batsch1788
Cryptodira Cope, Reference Cope1868
Chelydroidea Baur, Reference Baur1893
Kinosternoidea Hutchison and Weems, Reference Hutchison and Weems1998
Dermatemydidae Baur, Reference Baur1888
Cardichelyon Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013
Type species
Cardichelyon rogerwoodi Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013, by original designation, by monotypy.
Cardichelyon rogerwoodi Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013
Figures 1–4
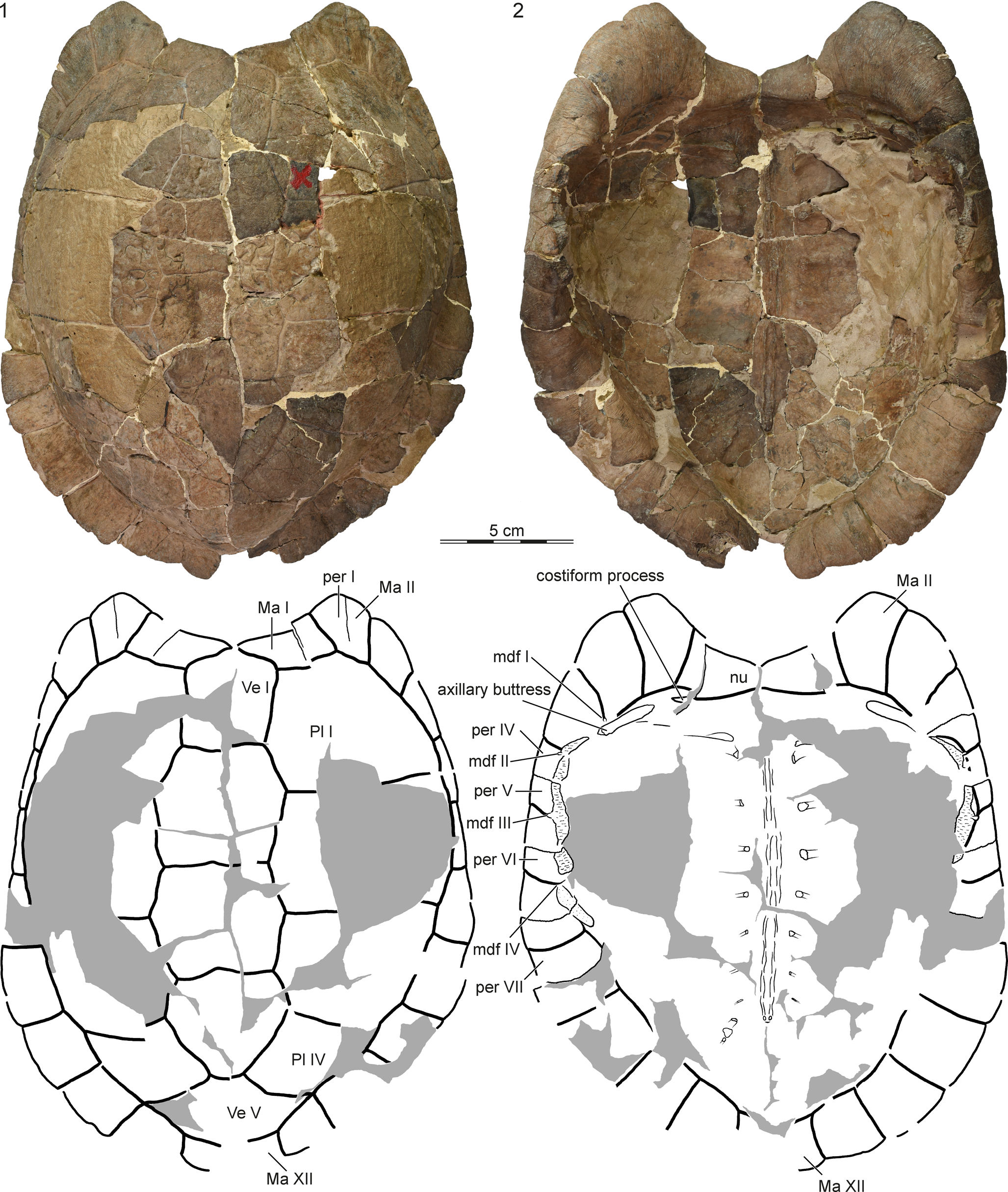
Figure 1. YPM PU14671, holotype of Cardichelyon rogerwoodi, late Paleocene (Clarkforkian) of Wyoming. Reconstructed carapace in (1) dorsal and (2) ventral view. Gray areas highlight reconstruction. Abbreviations: Ma = marginal scute; mdf = musk duct foramen; nu = nuchal; per = peripheral; Pl = pleural scute; Ve = vertebral scute.

Figure 2. YPM PU14671, holotype of Cardichelyon rogerwoodi, late Paleocene (Clarkforkian) of Wyoming. Detailed view of (1) the left costiform process and axillary buttress and (2) the left bridge peripherals. Abbreviations: Ma = marginal scute; nu = nuchal; per = peripheral.

Figure 3. YPM PU14671, holotype of Cardichelyon rogerwoodi, late Paleocene (Clarkforkian) of Wyoming. Reconstructed plastron in (1) ventral, (2) dorsal, and (3) and anterior view. Gray areas highlight reconstruction. Abbreviations: Ab = abdominal scute; An = anal scute; ent = entoplastron; epi = epiplastron; Fe = femoral scute; Gu = gular scute; Hu = humeral scute; hyo = hyoplastron; hyp = hypoplastron; Pe = pectoral scute; xi = xiphiplastron.

Figure 4. YPM PU16443, Cardichelyon rogerwoodi, late Paleocene (Clarkforkian) of Wyoming. Plastron in ventral view. Abbreviations: Ab = abdominal scute; An = anal scute; ent = entoplastron; epi = epiplastron; Fe = femoral scute; Gu = gular scute; Hu = humeral scute; hyo = hyoplastron; hyp = hypoplastron; Pe = pectoral scute; xi = xiphiplastron.
Holotype
YPM PU14671, a partial skeleton, including a restored shell, a partial skull, and fragments of long bones and girdles (Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013, figs. 26.8, 26.12, 26.13; Figs. 1–3) from Reis Quarry, Park County, Wyoming, USA, Fort Union Formation, Clarkforkian NALMA, Thanetian, late Paleocene (Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013).
Diagnosis
See Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) and Vlachos (Reference Vlachos2018).
Occurrence
Late Paleocene (Clarkforkian NALMA) to early Eocene (Wasatchian) of Wyoming (Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013).
Description
In addition to the description given below, see Figures 1–4.
Carapacial bones
The carapace of the holotype (YPM PU14671) is almost completely fused (Fig. 1), we therefore cannot discern the arrangement of neural and costal bones in this specimen. Illustrations of referred specimens provided by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013, fig. 26.8, 26.11), however, suggest that the carapace consists of a nuchal, eight often irregular neurals, two suprapygals, a pygal, eight pairs of costals that lack a midline contact, and 11 pairs of peripherals.
A number of important characters can be observed on the inside of the carapace that have not been highlighted previously (Figs. 1, 2). The attachment site of dorsal rib I is short and the rib heads of dorsal ribs II–IX are notably slender. It is unclear if the proximal end of dorsal rib X was ossified in life because this part of the shell is not preserved. As previously noted by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013), a groove is formed at the junction of costal I with peripherals II and III that holds the rib-like axillary buttress of the hyoplastron (Fig. 2.1). As also noted by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013), the rib of costal I inserts distally into peripheral IV, just below the axillary buttress. However, the nuchal forms a small, previously unnoted costiform process that penetrates the medial third of peripheral I (Fig. 2.1).
We agree with Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) that the bridge reaches from the anterior part of peripheral IV to the midpoint of peripheral VI, but we note a previously undocumented shift in the articulation with the plastron at peripheral VI (Figs. 1, 2.2). In particular, while the articular site with the anterior plastral lobe protrudes medially, is notably thick, and shows evidence of a former sutural contact with the hyoplastron, the articular site with the posterior plastral lobe is recessed, has a granular texture, and lacks interdigitations with the hypoplastron. This morphology is consistent with a kinetic posterior plastral lobe, which is confirmed by the morphology of the plastron (see below). We agree with Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) that peripherals III–VI form four pairs of musk duct foramina. The first musk duct is located on peripheral III at the intersection of marginals III and IV with the skin sulcus. The second and third musk duct foramina are positioned on peripherals IV and V at the intersections of marginals IV, V, and VI with the bridge. The fourth musk duct foramen on peripheral VI is only poorly developed because it coincides with the hinge line of the plastron.
Carapacial scutes
We agree with Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) that the carapace of Cardichelyon rogerwoodi is covered by five vertebrals, four pairs of pleurals, and 12 pairs of marginals (Fig. 1). The holotype and a referred specimen figured by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013, fig. 26.9) apparently lack a cervical. Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) noted that a minute cervical may be present in unfigured specimens, but we are not able to confirm this observation for the moment. Vertebral I is a narrow, pentagonal element with short anterolateral contacts with marginal I, broad lateral contacts with pleural I, and a straight posterior contact with vertebral II. Vertebrals II and III are hexagonal and slightly broader than long. Vertebral IV is pentagonal, with longer posterolateral sides. Vertebral V is hexagonal, but the anterior sulcus with vertebral III is much broader than the posterior sulcus with vertebral V. Vertebral V is the broadest scute in the vertebral series. It has a short anterior contact with vertebral IV, a broad anterolateral contact with pleural IV, a point contact with marginal X, and broad posterior contacts with marginals XI an XII. The location of the intervertebral sulcus relative to the neural column is obscured in the holotype, but illustrations of referred specimens provided by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013, fig. 26.11) suggest that the vertebral I/II sulcus is located on neural I, the vertebral II/III sulcus on neural III, the vertebral III/IV sulcus on neural V or VI, and the vertebral IV/V sulcus on neural VIII or suprapygal I. The low and narrow nature of the marginals suggests that they are restricted to the peripherals.
Plastral bones
We generally agree with the morphology of the plastron as presented by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013, fig. 26.12). As in all other cryptodires, the plastron consists of an entoplastron, and a pair of epi-, hyo-, hypo-, and xiphiplastra (Figs. 3, 4). The plastron is notable for being relatively thick (~7 mm at the center) and for having deep and thick lips along the margins of the anterior and posterior lobes (up to 1.5 cm). In anterior view, the epiplastra jointly form a deep trough (Fig. 3.3).
The hyoplastron has a sutural contact with the posterior three-quarters of peripheral IV, peripheral V, and the anterior third of peripheral VI and forms an elongate axillary buttress that is located along the suture of costal I with peripherals II and III. The anterior plastral lobe was therefore fully immobilized. The hypoplastron, by contrast, forms a short inguinal buttress that ligamentously attached to the posterior two-thirds of peripherals VI and the anterior half of peripheral VII. The posterior plastral lobe was therefore kinetically articulated with the rest of the shell. The type plastron is reconstructed as having a broad posterior plastral lobe and a blunt anal notch (Fig. 3), but a previously unfigured plastron from the type locality (YPM PU16443; Fig. 4) highlights a more distinct anal notch. Although we cannot exclude intraspecific variation, large amounts of plaster lead us to believe that the anal notch of the type specimen was reconstructed incorrectly. We note the presence of paired fields of crenulations with unknown function on the visceral side of the hypoplastron anteromedially to the inguinal notches in the type specimens (Fig. 3).
Plastral scutes
We concur with Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) that the plastron is covered by a pair of gular, humeral, pectoral, abdominal, femoral, and anal scutes with midline contacts (Figs. 3, 4). Inframarginals appear to be absent. In contrast to the referred plastron figured by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013, fig. 26.12.c), the gulars and pectorals of the holotype barely overlap the anterior and posterior tips, respectively, of the entoplastron in the holotype. The pectoral/abdominal sulcus closely approximates the hyo/hypoplastral suture. In addition, the portion of the abdominal that overlaps the hyoplastron has a finely crenulated surface. A similar arrangement is found in extant testudinoids with hinges with limited mobility. All scutes jointly cover the visceral side of the plastron to form broad plastral lips (Fig. 3). These lips are at their thickest along the gulars and the femorals.
Remarks
None.
Phylogenetic analysis
Our character analysis (see Discussion below) highlights that Cardichelyon rogerwoodi possesses characters that either suggest relationships with Testudinoidea or Chelydroidea (i.e., the clade consisting of snapping turtles [Chelydridae] and mud and musk turtles [Kinosternoidea]). We are not able to test either position in a global context because no analysis of turtle relationships is currently available that densely samples these two groups of turtles and the characters that diagnose them. Because we herein ultimately favor relationships of this turtle with Chelydroidea (see Discussion below), we inserted Cardichelyon rogerwoodi into the chelydroid analysis of Lyson et al. (Reference Lyson, Joyce and Sertich2017), an expansion of the kinosternoid matrix of Knauss et al. (Reference Knauss, Joyce, Lyson and Pearson2011), to investigate its possible placement within the clade Chelydroidea. All characters were scored based on the two specimens described herein (YPM PU14671, the holotype, and YPM PU16443), with exception of the neural and cervical characters, which were scored based on material described by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013). We furthermore added four testudinoids to the matrix to explore their impact on the analysis, in particular the Late Cretaceous Mongolemys sp. (based on material referred to Mongolemys spp. by Danilov, Reference Danilov2001), the extant emydid Chrysemys picta (Schneider, Reference Schneider1783) (AMNH 75250), the extant testudinid Gopherus agassizii (Cooper, Reference Cooper1861) (USNM 222094), and the extant platysternid Platysternon megacephalum Gray, Reference Gray1831 (FMNH 51627). The analysis was furthermore expanded to include three additional shell characters that could eventually be relevant for differentiating testudinoids from chelydroids: character 66, number of musk glands (0 = none; 1 = one; 2 = two; 3 = three; 4 = four); character 67, costal contact of axillary buttresses (0 = absent; 1 = present); and character 68, costal contacts of inguinal buttresses (0 = absent; 1 = present). All extant taxa were scored for character 66 by reference to Waagen (Reference Waagen1972). The remaining characters were scored using the same resources as Knauss et al. (Reference Knauss, Joyce, Lyson and Pearson2011) and Lyson et al. (Reference Lyson, Joyce and Sertich2017). The final characters matrix consisting of 68 characters scored for 29 taxa is provided in Supplementary data set 1.
The matrix was subjected to a parsimony analysis using TNT (Goloboff et al., Reference Goloboff, Farris and Nixon2008) with Judithemys sukhanovi Parham and Hutchison, Reference Parham and Hutchison2003 selected as the outgroup. Eleven characters form morphoclines and were run ordered (i.e., characters 4, 10, 16, 22, 25, 29, 34, 40, 44, 45, 66). Following the recommendation of Goloboff et al. (Reference Goloboff, Torres and Salvador Arias2018), light implied weighting was implemented using a K-factor of 12. The matrix was subjected to 1000 replicates of random addition sequences followed by a second round of tree bisection-reconnection. Two primary analyses were performed that differ in their inclusion of the four testudinoids. Because the testudinoids did not resolve themselves as suggested by recent molecular analyses (e.g., Pereira et al., Reference Pereira, Sterli, Moreira and Schrago2017), we forced the sister group relationship of the fossil Mongolemys spp. to all extant testudinoids, and the sister group relationship between the emydid Chrysemys picta and Platysternon megacephalum relative to Gopherus polyphemus through the use of a backbone constraint. All remaining taxa were left to float. The analysis that includes the testudinoids resulted a single tree as well with a score of 8.26752, while the analysis to the exclusion of the four testudinoids resulted in a single tree with a score of 6.49208. These trees are provided in Figure 5.
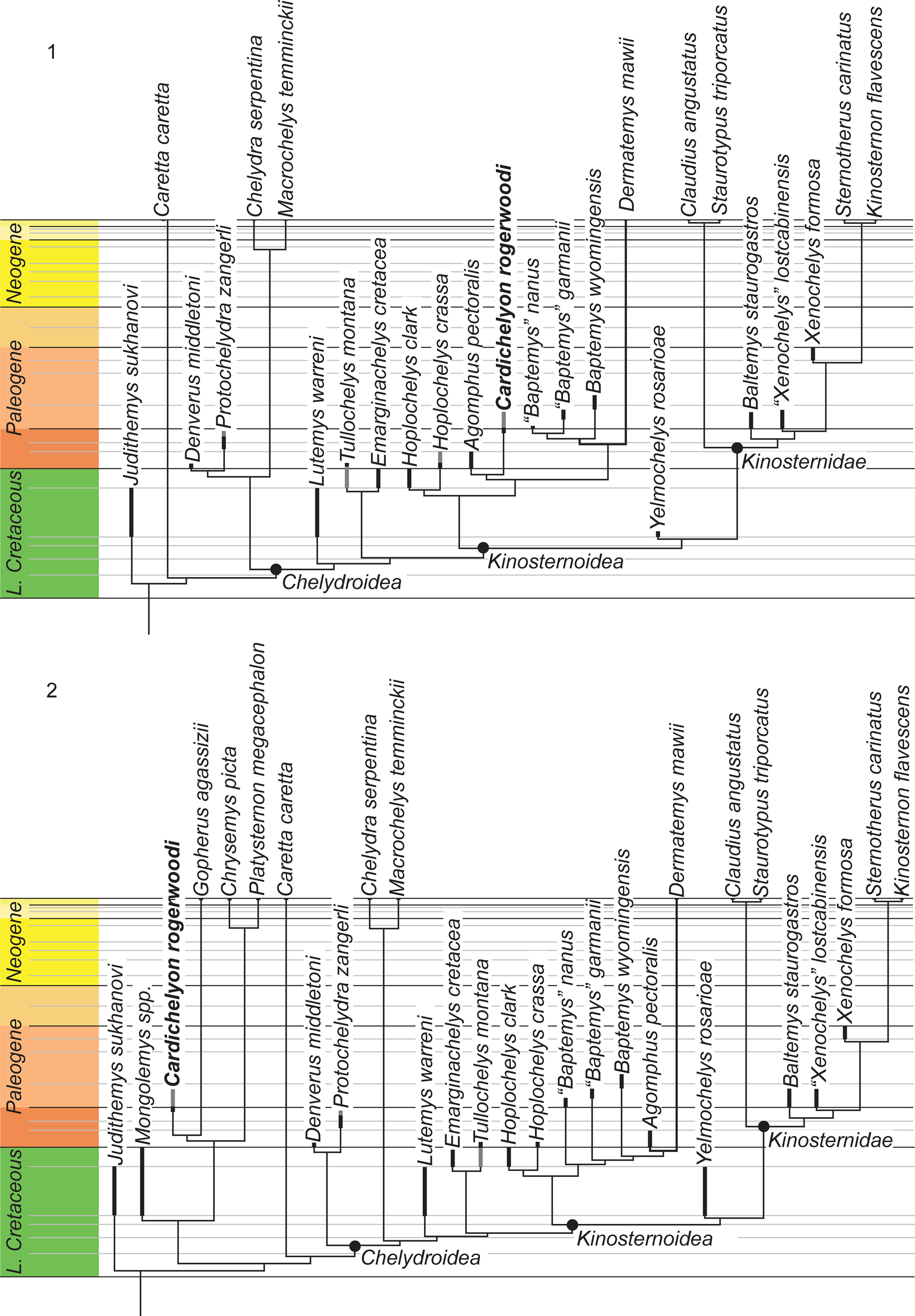
Figure 5. The time calibrated trees retrieved from the phylogenetic analyses performed herein that differ in the (1) inclusion and (2) exclusion of testudinoids. Thick dark bars denote the type range of a particular species, gray bars connote the range of referred material.
Discussion
Cardichelyon rogerwoodi exhibits a number of conflicting characters that either suggest chelydroid or testudinoid affinities. As characters are used to initially assess the phylogenetic affinities of taxa, we list in the first section of this discussion the most notable characters apparent in this turtle and examine their currently known distribution across the tree to assess their phylogenetic significance. In the next section, we then discuss the results of our phylogenetic analysis. In the final section, we use consilience to argue for the chelydroid affinities of this unusual turtle.
Character analysis
Prior to its formal description, Cardichelyon rogerwoodi was thought to be an emydid or a geoemydid (Estes, Reference Estes1975; Bartles, Reference Bartels1980, Reference Bartels1983; Hutchison, Reference Hutchison1980, Reference Hutchison, Prothero and Berggren1992, Reference Hutchison, Aubry, Lucas and Berggren1998, Reference Hutchison, Brinkman, Holroyd and Gardner2013; McCord, Reference McCord1996), but no character evidence was provided to support either assessment. Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) suggested platysternid affinities for this taxon based on its macrocephalic nature and presence of multiple musk duct foramina. This was informally accepted by Vlachos (Reference Vlachos2018), but the format of this contribution prohibited him from exploring the phylogenetic relationships of this enigmatic turtle more extensively. In all cases, however, previous authors presumed this taxon to have testudinoid affinities.
The shell of Cardichelyon rogerwoodi possesses a number of characters that indeed are consistent with testudinoid relationships, but also with kinosternoid relationships. We here briefly highlight and discuss the most important ones.
Plastral scutes
Testudinoids are unique among extant turtles by possessing paired gulars, humerals, pectorals, abdominals, femorals, and anals (Hutchison and Bramble, Reference Hutchison and Bramble1981), in contrast to extant chelydrids and kinosternoids, which possess intergulars, but lack pectorals (Joyce, Reference Joyce2016; Joyce and Bourque, Reference Joyce and Bourque2016). A recent phylogenetic analysis of Americhelydia with focus on Chelydroidea (i.e., the clade consisting of Chelydridae and Kinosternoidea) concluded that the lack of pectorals is a synapomorphy of Chelydroidea, but that pectorals were secondarily reacquired within this clade, for instance in the Paleocene dermatemydid Agomphus pectoralis (Cope, Reference Cope1868) (Lyson et al., Reference Lyson, Joyce and Sertich2017). Similarly, although the presence of intergulars may optimize to be a synapomorphy of Americhelydia or Chelydroidea, these scutes are lacking in numerous forms, including most dermatemydids. In addition to Cardichelyon rogerwoodi, the testudinoid arrangement of plastral scutes is also found in the tentative kinosternoid Planetochelys dithyros Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013 (Joyce and Bourque, Reference Joyce and Bourque2016). The plastral formula seen in Cardichelyon rogerwoodi is therefore consistent with both kinosternoid and testudinoid relationships.
Extensive plastral lips
In contrast to extant americhelydians, most testudinoids are typically characterized by the presence of extensive plastral lips (i.e., broad margins along the visceral sides of the anterior and posterior plastral lobes covered by scutes). However, extensive plastral lips are also present in many other turtles, particularly those with broad plastra, including the extant pelomedusid Pelusios castanoides Hewitt, Reference Hewitt1931 (BMNH 1911.4.7.2), the chelid Platemys platycephala (Schneider, Reference Schneider1792) (FMNH 45659), and fossil nanhsiungchelyids, such as “Zangerlia” dzamynchondi Sukhanov and Narmandkh, Reference Sukhanov and Narmandakh2006 (Danilov et al., Reference Danilov, Sukhanov, Syromyatnikova, Brinkman, Holroyd and Gardner2013). In addition to Cardichelyon rogerwoodi, we also note the presence of plastral lips in the tentative kinosternoid Planetochelys dithyros Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013, the stem kinosternid Xenochelys lostcabinensis Hutchison, Reference Hutchison1991, and the extant kinosternid Kinosternon flavescens Agassiz, Reference Agassiz1857 (AMNH 73826). This character is therefore consistent with both kinosternoid and testudinoid affiliations.
Plastral kinesis
Plastral kinesis (i.e., a plastral hinge) has evolved multiple times across the turtle tree. The independent acquisition of hinges is suggested in part by the non-homologous structures that are involved. For instance, an epi/hyoplastral hinge is found in the kinosternid Kinosternon, an anteriorly mobile hyo/hypoplastral hinge in the pelomedusids Pelusios, a posteriorly mobile hyo/hypoplastral hinge in the fossil testudinoid Ptychogaster, a fully mobile hyo/hypoplastral hinge in the emydid Cuora, and a hypo/xiphiplastral hinge again in the kinosternid Kinosternon (e.g., Bramble, Reference Bramble1974; Mlynarski, Reference Mlynarski1976; Bramble and Hutchison, Reference Bramble and Hutchison1981; Bramble et al., Reference Bramble, Hutchison and Legler1984). Although a hyo/hypoplastral suture has not previously been reported for a kinosternoid, with exception of the tentative kinosternoid Planetochelys dithyros, the great number of independent acquisitions of this character complex does not provide much support for any particular clade.
Musk glands
Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) noted that the presence of multiple musk duct glands suggests platysternid relationships for Cardichelyon rogerwoodi, but we presume that this assessment was made under the assumption that this fossil is a testudinoid. In general, testudinoids only possess two pairs of musk glands, an axillary gland located near the junction of marginals III and IV and an inguinal gland near the junction of marginals VI and VII (Waagen, Reference Waagen1972). An important exception to the rule is Platysternon megacephalum, which possesses doubled axillary glands associated with marginals IV and V, in addition to an inguinal gland, an arrangement also seen in some individuals of Terrapene (Waagen, Reference Waagen1972). The musk ducts of Platysternon megacephalum do not leave any trace in the bony structures surrounding the ligamentous bridge. The four preserved musk duct foramina of Cardichelyon rogerwoodi located at the junctions of marginals III–VII therefore do not align well with those of Platysternon megacephalum.
Chelydrids possess four pairs of glands that are situated mid-body associated with marginals V–VII. Kinosternoids, in contrast, possess two pairs of glands, of which the anterior is situated far to the front associated with marginal III and the posterior at the back associated with marginal VII (Waagen, Reference Waagen1972). Cardichelyon rogerwoodi appears to possess a condition intermediate between that of extant chelydrids and kinosternoids by possessing more than two pairs of glands, as in chelydrids, of which the most anterior is displaced towards the front, as in kinosternoids. The musk duct foramina of Cardichelyon rogerwoodi are therefore overall more consistent with it being a chelydroid.
Costiform processes
We here note for the first time that Cardichelyon rogerwoodi possesses short costiform processes (i.e., lateral projections formed by the nuchal that protrudes laterally into the peripherals). Although one of us (WGJ) previously argued that costiform processes are restricted to chelydroids (Joyce, Reference Joyce2007; Lyson et al., Reference Lyson, Joyce and Sertich2017), we here side with Shaffer et al. (Reference Shaffer, Meylan and McKnight1997) by noting the presence of short costiform processes not only in some individuals of the testudinoid Platysternon megacephalum, but also some nanhsiungchelyids, such as Hanbogdemys orientalis (Sukhanov and Narmandakh, Reference Sukhanov and Narmandakh1975) (Sukhanov, Reference Sukhanov, Benton, Shishkin, Unwin and Kurochkin2000). Costiform processes are therefore typical of chelydroids, but not unique to the group.
Rib-like axillary processes
The hyoplastron forms elongate axillary processes in a series of turtles to stabilize the bridge, but its shape and contacts have phylogenetic significance. Although the length of the axillary processes varies greatly in testudinoids, if present, it typically curves towards the midline at peripheral III towards dorsal ribs I and II to contact costal I (Joyce and Bell, Reference Joyce and Bell2004). Among chelydroids, elongate axillary processes are only present in kinosternoids, particularly those associated with the Dermatemys lineage. In these taxa, the axillary buttress forms an elongate, rib-like process that runs along the peripheral/costal contact towards the costiform processes of the nuchal and does not insert distally along the visceral side of costal I (Knauss et al., Reference Knauss, Joyce, Lyson and Pearson2011). In this regard, the rib-like axillary buttress of Cardichelyon rogerwoodi more closely matches the axillary buttress of kinosternoids, in particular the Dermatemys lineage, rather than that of testudinoids.
Anal notch
A notable feature of Cardichelyon rogerwoodi is its deep anal notch. Among testudinoids, deep anal notches are present in geoemydids and testudinids, but absent in emydids and platysternids. Well-developed anal notches are also present in some kinosternoids, such as the late Eocene Xenochelys formosa Hay, Reference Hay1906 or the extant Dermatemys mawii Gray, Reference Gray1847 (FMNH 98950). This character therefore does not have a strong phylogenetic signal.
In conclusion, the presence of pectorals, extensive plastral lips, hyo/hypoplastral kinesis, a deep anal notch, and the absence of intergulars are typical of testudinoids, but their homoplastic presence among kinosternoids is notable. Costiform processes are consistently developed in kinosternoids, but are known to occur homoplastically elsewhere, including testudinoids. The rib-like axillary process and association of a musk duct foramen with marginal III are typical of kinosternoids, but there are no reasons a priori to conclude that they cannot have developed homoplastically in other groups as well. Cardichelyon rogerwoodi is therefore either an unusual testudinoid with supernumerary, anteriorly shifted musk duct foramina, rib-like axillary processes, and costiform processes, or an unusual kinosternoid with hyo/hypoplastral hinge, pectoral scutes, and a deep anal notch.
Phylogenetic relationships
Our character analysis suggests that Cardichelyon rogerwoodi most plausibly represents an unusual testudinoid, or an unusual kinosternoid, but we are not able to test either hypothesis rigorously because we are not aware of an appropriate character/taxon matrix that scores these groups and their characters densely, and because constructing such a matrix is far beyond the scope of this contribution. We conclude below that consilience favors kinosternoid relationships, therefore we inserted Cardichelyon rogerwoodi into the chelydroid matrix of Lyson et al. (Reference Lyson, Joyce and Sertich2017), but performed two analyses that differ in the inclusion of testudinoids.
The analysis including testudinoids retrieves Cardichelyon rogerwoodi outside of Americhelydia within Testudinoidea, in particular as sister to Gopherus agassizii, not Platysternon megacephalum, as had been suggested previously (Fig. 5.1). However, because the matrix of Lyson et al. (Reference Lyson, Joyce and Sertich2017) was developed specifically to explore chelydroid relationships, we do not give weight to this particular placement within Testudinoidea. Interestingly, every single testudinoid by itself is sufficient to force the alternative topology. The placement of Cardichelyon rogerwoodi as sister to Gopherus agassizii is suggested by a single character, the presence of a relatively domed shell (21). Three additional characters (presence of a transverse epi/hyoplastral suture [32], presence of a reduced cervical [60], and presence of a costal contact of the axillary buttress [67]) support placement within Testudinoidea. Cardichelyon rogerwoodi itself possess seven autapomorphies (4, 8,12, 13, 21, 47, 66), none of which is a reversal of testudinoid characters. The result of this analysis differs from that of Lyson et al. (Reference Lyson, Joyce and Sertich2017) by placing Denverus middletoni Hutchison and Holroyd, Reference Hutchison and Holroyd2003 and Protochelydra zangerli Erickson, Reference Erickson1973 at the base of Chelydroidea and not as sister to Chelydridae.
The second analysis excluding testudinoids results in a single tree that resembles the result of Lyson et al. (Reference Lyson, Joyce and Sertich2017) by hypothesizing an extended dermatemydid lineage consisting not only of Hoplochelys and Baptemys, but also of Agomphus pectoralis and Cardichelyon rogerwoodi (Fig. 5.2). In contrast to Lyson et al. (Reference Lyson, Joyce and Sertich2017), however, Baptemys is reconstructed as monophyletic, which implies an extended ghost lineage for Dermatemys mawii. The sister group relationship of Agomphus pectoralis and Cardichelyon rogerwoodi is supported by the insertion of costal rib I into peripheral VI (8) and the presence of pectorals (28). Cardichelyon rogerwoodi is placed well within the pan-kinosternoid clade in this analysis. In particular, it is placed within Dermatemydidae, supported by five characters (13, 22, 29, 30, 67). More generally, its placement within Pan-Kinosternoidea is supported by another 11 characters (26, 27, 40, 44–47, 52, 53, 62, 64). Cardichelyon rogerwoodi itself exhibits seven autapomorphies (12, 15, 18, 20, 23, 29, 32), but none of these is a reversal of the characters that nest it within Pan-Kinosternoidea. This final observation is consistent for taxa naturally nested deep within the tree because terminal taxa incorrectly pulled into the tree by homoplasy display high numbers of reversals.
Consilience
Even though parsimony suggests that Cardichelyon rogerwoodi has testudinoid relationships (see previous section), three external lines of evidence (time, biogeography, and phylogenetic context) favor chelydroid relationships instead.
Time and biogeography
As currently understood, a number of Asian lineages (carettochelyids, testudinids, geoemydids, and, perhaps, emydids) appeared in North American nearly instantaneously at the Paleocene/Eocene boundary, likely as a response to a sudden shift in biomes during the Paleocene/Eocene Thermal Maximum (PETM; Hutchison, Reference Hutchison, Aubry, Lucas and Berggren1998; Joyce et al., Reference Joyce, Rabi, Clark and Xu2016; Vlachos, Reference Vlachos2018). Although the Behring Land Bridge consistently existed during the Paleogene, unknown barriers apparently hindered turtles from crossing it. Asian faunas arrived in Europe either directly from Asia or from North America following the PETM as well (e.g., Claude and Tong, Reference Claude and Tong2004; Lourenço et al., Reference Lourenço, Claude, Galtier and Chiari2012; Smith et al., Reference Smith, Quesnel, De Ploëg, De Franceschi, Métais, De Bast, Solé, Folie, Boura, Claude, Dupuis, Gagnaison, Iakovleva, Martin, Maubert, Prieur, Roche, Storme, Thomas, Tong, Yans and Buffetaut2014). This general pattern would not hold true if Cardichelyon rogerwoodi were a testudinoid because this turtle would have crossed the Behring Land Bridge long before the climatic event that allowed the coordinated dispersal of its relatives (Vlachos, Reference Vlachos2018), as early as the Tiffanian (Hutchison, Reference Hutchison, Brinkman, Holroyd and Gardner2013). In contrast, if Cardichelyon rogerwoodi is a chelydroid, no uncoordinated dispersal event is needed to explain its presence in the Paleocene fossil record of North America because this clade was endemic to this continent for most of its history (Joyce, Reference Joyce2016; Joyce and Bourque, Reference Joyce and Bourque2016).
Phylogenetic context
Our phylogenetic analyses suggest two primary hypotheses for the placement of Cardichelyon rogerwoodi: either within Testudinoidea or as sister to Agomphus pectoralis at the base of Dermatemydidae. Either hypothesis is made more meaningful by the biogeographic and temporal patterns it explains and the character evolution it implies. The testudinoid hypothesis does not have much explanatory power because it suggests the isolated occurrence of a hinged testudinoid in the late Paleocene of North America that fortuitously shares numerous unusual characteristics (e.g., costiform processes, rib-like axillary processes, supernumerary musk glands) with unrelated, but contemporary turtles from North America. The dermatemydid hypothesis, on the other hand, embeds Cardichelyon rogerwoodi in the kinosternoid tree in a meaningful way because this turtle is placed in close association with Agomphus pectoralis or Hoplochelys crassa (Cope, Reference Cope1888), two roughly coeval taxa from North America that happen to share the abovementioned characteristics. We therefore favor this hypothesis herein.
Outlook
Our conclusion that consilience favors Cardichelyon rogerwoodi as a kinosternoid provides testable predictions in regards to the morphology of elements not yet found. The skeleton of chelydroids differs most consistently perhaps from that of testudinoids in the morphology of the cervical vertebrae. Although much variation is apparent, testudinoids typically show double articulations between cervicals V–VIII and they have a biconvex cervical IV and a biconcave cervical VII. In the cervical column of chelydroids, by contrast, double articulations are restricted to cervicals VI–VIII, and cervical II or III are often, though not always, biconvex, particularly in dermatemydids, and the posterior cervicals are consistently procoelous (Williams, Reference Williams1950). These two groups of turtles further differ in the morphology of their pectoral and pelvic girdles because many kinosternoids exhibit an accessory process on the scapula, a true thelial process, and a deep ilial notch (Lyson et al., Reference Lyson, Joyce and Sertich2017), although we suspect that the pelvis of Cardichelyon rogerwoodi is modified to accommodate for the biomechanical demands of a posterior plastral hinge (Bramble, Reference Bramble1974). Even a complete skull of Cardichelyon rogerwoodi may not provide much useful data, given that the partial skull described by Hutchison (Reference Hutchison, Brinkman, Holroyd and Gardner2013) appears to be highly modified, much as the highly modified skull of the testudinoid Platysternon megacephalum, which was incorrectly argued to be a chelydrid based on skull characters (Gaffney, Reference Gaffney1975; Parham et al., Reference Parham, Feldman and Boore2006; Joyce and Sterli, Reference Joyce and Sterli2012).
Acknowledgments
We would like to thank D. Brinkman and J. Gauthier (YPM) for an extended loan of the type specimens of Cardichelyon rogerwoodi; E. Ascarrunz and R. Garbin provided useful discussions; J. Parham and an anonymous reviewer are thanked for critical comments that significantly helped improve this manuscript. This project was funded by grants from the Swiss National Science Foundation to WGJ (SNF 200021_153502/1, 2).
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.sn02v6×0t.







