Introduction
Ongoing research of the geology and paleontology of the late Tertiary basins in the central part of Mexico, between latitudes 23°N and 19°N, has been pivotal to expanding our knowledge of Hemphillian and Blancan North American Land Mammal age (NALMA) faunas found to the south of the classic localities of these ages in the North American Great Plains. The faunas recovered to date have a high mammalian diversity and represent the most important late Cenozoic faunal assemblages known south of the United States border. All of the sites that have been studied and collected consist of fluvial lacustrine deposits that form part of the Trans Mexican Volcanic Belt, and contain the earliest appearances of South American immigrants that dispersed into North America during the Hemphillian and Blancan (9.0–1.6 Ma).
The ages of these fossil records have been obtained by both radiometric and paleomagnetic analyses. These have refined our knowledge of both the earliest existence of the Panamanian land bridge that was critical for the Great American Biotic Interchange (GABI) and the timing of the dispersal of taxa prior to its formation (Carranza and Miller, Reference Carranza and Miller1980; Miller and Carranza-Castañeda, Reference Miller and Carranza-Castañeda2001; Carranza-Castañeda and Miller, Reference Carranza-Castañeda and Miller2004; Flynn et al., Reference Flynn, Kowallis, Nuñez, Carranza-Castañeda, Miller, Swisher and Lindsay2005; Woodburne, Reference Woodburne2010; Carranza-Castañeda et al., Reference Carranza-Castañeda, Aranda-Gómez, Wang and Iriondo2013).
During the early Blancan, the northward dispersal of South American neotropical mammals into North America was more dynamic and involved more taxa than the earlier dispersal in the Hemphillian (~8 Ma). Evidence of this active interchange has been recorded at several localities in the San Miguel Allende Basin, in the state of Guanajuato. These important fossil sites encompass faunas from the latest Hemphillian, dated at 4.8 Ma, as well as various early Blancan localities, dated at 4.7 Ma. We note that the Hemphillian-Blancan boundary as used here (4.8–4.7 Myr; Flynn et al., Reference Flynn, Kowallis, Nuñez, Carranza-Castañeda, Miller, Swisher and Lindsay2005) is later than that of Lindsay et al. (Reference Lindsay, Mou, Downs, Pederson, Kelly, Henry and Trexler2002) in which this boundary was defined as 4.9–5.0 Ma based on stratigraphic sequences in Nevada. The Mexican faunas document the earliest records in North America of taxa originating in the neotropics of South America: Glyptotherium texanum Osborn, Reference Osborn1903 (Gillette et al., Reference Gillette, Carranza-Castañeda, White, Morgan, Thrasher, McCord and McCullough2016), Paramylodon garbani (Montellano-Ballesteros and Carranza-Castañeda, 1981, Reference Montellano-Ballesteros and Carranza-Castañeda1986), and Neochoerus cordobae Carranza-Castañeda and Miller, Reference Carranza-Castañeda and Miller1988 (Miller and Carranza, Reference Miller and Carranza-Castaneda1998; Miller and Carranza-Castañeda, Reference Miller and Carranza-Castañeda1999; Carranza-Castañeda and Miller, Reference Carranza-Castañeda and Miller2004; Flynn et al., Reference Flynn, Kowallis, Nuñez, Carranza-Castañeda, Miller, Swisher and Lindsay2005; Carranza-Castañeda, Reference Carranza-Castañeda2016). All of these records strongly suggest that the second stage of the GABI occurred at least 1.5 Myr earlier than had been previously accepted based on the presence of these taxa in faunas farther north in Arizona, Texas, and Florida.
Results of additional studies in other basins in central Mexico include localities in the State of Hidalgo, such as the Las Golondrinas locality, an important late Hemphillian fauna that includes Megalonyx, as well as the Zietla fauna from which several megalonychid molariforms were recovered (Carranza-Castañeda, Reference Carranza-Castañeda1991).
Studies carried out in the state of Jalisco have revealed the significance of the Tecolotlán Basin to our understanding the early stages of the GABI in Mexico. Here the stratigraphic sequence includes an important late Hemphillian fauna in the upper part of the unit, above which rest unconformably younger beds that have been assigned to the late Blancan–early Irvingtonian NALMA, and within this sequence, South American immigrants have been discovered (Kowallis et al., Reference Kowallis, Miller, Carranza-Castañeda, Christiansen, Swisher, Ross, Deino and Tingey2003, Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017; Carranza-Castañeda, Reference Carranza-Castañeda2016).
Materials and methods
The specimen described here was collected and prepared with regular procedures. All the measurements are in mm. The specimen was compared with a cast of the jaw of Megalonyx curvidens Matthew, Reference Matthew1924, the jaw of Pliometanastes from Rio Vírgenes, México and housed in the LACM collections, and Pliometanastes from the Siphon Canal locality, California. The photograph of the longitudinal section of the labial side of the jaw, was made with X-Ray microtomography at the Laboratorio Universitario de Microtomografía de Rayos X (LUMIR), del Centro de Geociencias, UNAM.
Initial analysis of the volcanic ash was by Ar39/Ar40 and yielded an age of 4.95±0.16 Ma (Kowallis et al., Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017). A new analysis of the volcanic ash based on U/Pb dating using laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) conducted in the Laboratorio de Estudios Isotópicos del Centro de Geociencias, UNAM produced an age of 4.85 ± 0.1 Ma.
Repositories and institutional abbreviations
Vertebrate remains were recovered from multiple localities, which are identified as Jal Teco followed by a number to indicate Jalisco-Tecolotlán. Institutional abbreviations include FAM=Frick Collection, American Museum of Natural History New York; LACM=Natural History Museum of Los Angeles County, Los Angeles; MACN=Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires; MPGJ=Museo de Paleontología Geociencias, Juriquilla; MPM=Museo Regional Provincial Padre M. J. Molina, Rio Gallegos; UF=Florida Museum of Natural History, Gainesville; UNAM=Universidad Nacional Autónoma de México, México City.
Tecolotlán geological setting
The Tecolotlán Basin is located ~80 km southwest of Guadalajara (Fig. 1). The fossil-bearing sediments that accumulated in the basin occupy a 20×10 km area, in a N to SE-trending depression, centered approximately at 20º10'N, 104º03'W, which is roughly parallel to the nearby Colima rift, located 50 km to the east. The continental sedimentary basin is near the northeast boundary of the Jalisco Block, which is a region bounded to the north by the WNW-trending Tepic-Zacoalco rift, to the east by the NE-trending Colima rift and to the west by the northern end of the Middle America trench (Rosas-Elguera et al., Reference Rosas-Elguera, Ferrari, Garduño-Monroy and Urrutia-Fucugauchi1996). Rocks in the Jalisco block are dominated by the 100–75 Ma Puerto Vallarta batholith, and large areas are covered by intermediate to mafic volcanic rocks related to the Trans Mexican volcanic belt are 6–3 Ma old in the area near Tecolotlán (Rosas-Elguera et al., Reference Rosas-Elguera, Ferrari, Garduño-Monroy and Urrutia-Fucugauchi1996; Gómez-Tuena et al., Reference Gómez-Tuena, Orozco-Esquivel and Ferrari2007). The origin of the Tecolotlán Basin has not been studied, but we note that on both sides of the elongated depression there exist several conspicuous NE-trending lineaments that suggest the existence of a late Miocene–Pliocene graben in the area. Extensive outcrops of Pliocene mafic lavas south of the town of Tecolotlán (Paizanni-Herrera et al., Reference Paizanni-Herrera, Ramirez-Romo, Gamez-Ordaz and Ávila-Lugo1999) suggest that drainage in the graben may have been blocked periodically by volcanic products.
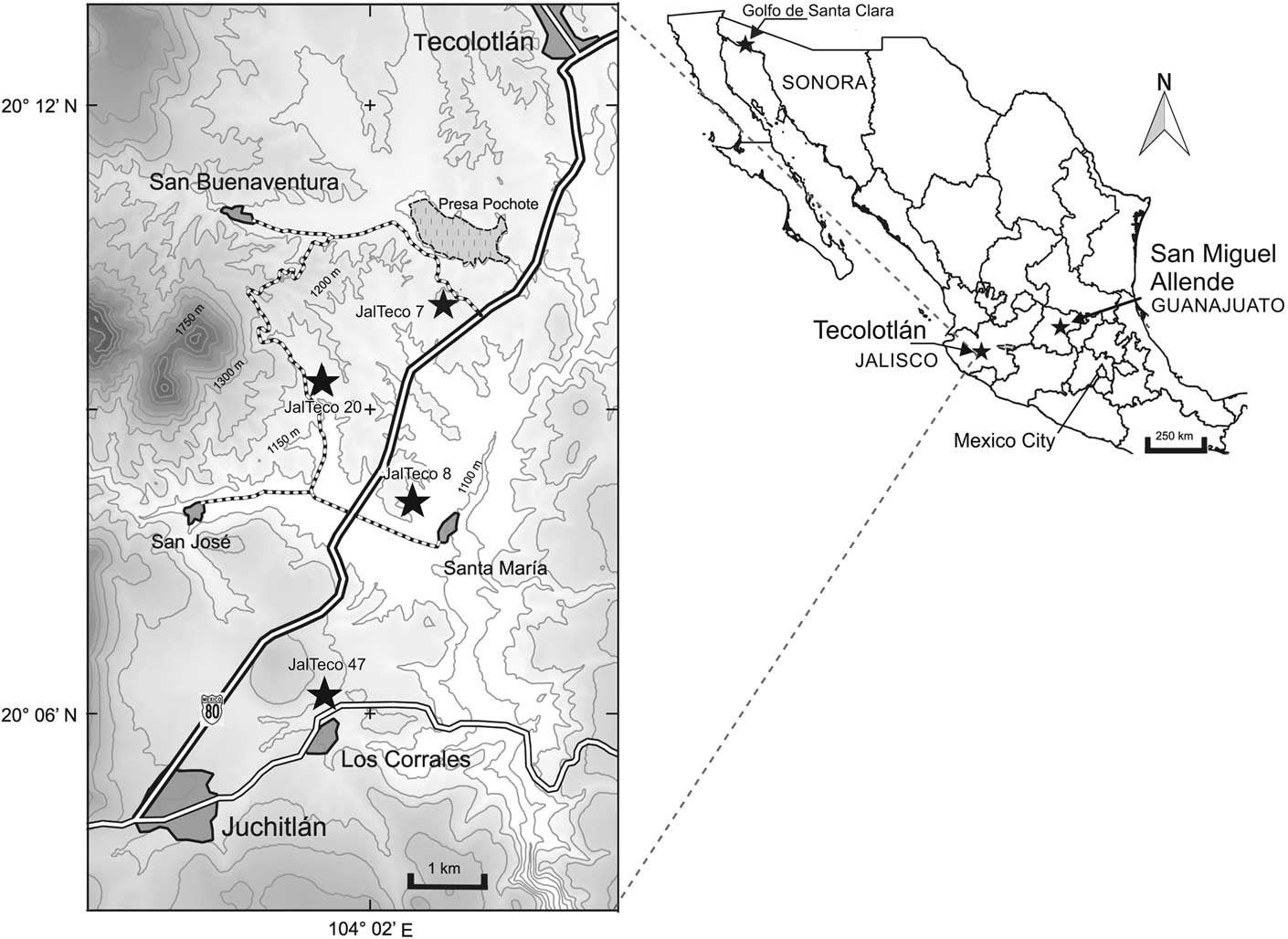
Figure 1 Map showing the location of sites in the Tecolotlán basin, Jalisco, Mexico cited in the text. The largest star is the type locality for Zacatzontli tecolotlanensis n. gen. n. sp.
The basin is delimited to the east by the oldest sedimentary rocks, limestones, exposed near San José de Los Guajes, and to the west by a series of interbedded late Cretaceous volcanic and sedimentary rocks informally called the Juchitlán beds (Kowallis et al., Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017).
Before 1997, there was no previous work related to the biostratigraphy of the Tecolotlán Basin. The first contributions were made by B. Kowallis of Brigham Young University (Kowallis et al., Reference Kowallis, Carranza-Castañeda, Miller, Toblen and Christiansen1998, Reference Kowallis, Miller, Carranza-Castañeda, Christiansen, Swisher, Ross, Deino and Tingey2003). Kowallis et al. (Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017) recently finished the most complete study of the area.
Stratigraphy
The Tecolotlán Basin is filled with fluvial-lacustrine sediments, consisting of poorly consolidated muds, clays, and sandstones that contain an abundance of North American mammalian faunas, many of which include South American immigrants, along with reptiles and fishes. Interbedded with these sediments are several important volcanic ashes that can be radiometrically dated. For the time being, this interesting late Hemphillian sequence has been informally called the San José beds (Kowallis et al., Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017).
Resting at the upper part of the sequence, above an erosional unconformity, is a more recent unit composed of sands, gravel, and sandy mudstones. This part of the section is named the San Buenaventura beds (Kowallis et al., Reference Kowallis, Miller, Carranza-Castañeda, Christiansen, Swisher, Ross, Deino and Tingey2003, Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017) and the age is assigned to the latest Blancan–Irvingtonian. In the southeast of the basin near the town of Juchitlán, another late Blancan deposit, Jal Teco 47 Los Pitahayos, has been described and contains the remains of two South American immigrant taxa: Glyptotherium cylindricum Brown, Reference Brown1912 and Neochoerus occidentalis Carranza Castañeda, Reference Carranza-Castañeda2016.
Because the distance between the late Hemphillian localities in which the fossil materials were collected is so great within the basin, for the purpose of this work, it was decided to consider two areas of major importance, taking into consideration that one third of the sequence is referred to the late Blancan (Fig. 2).
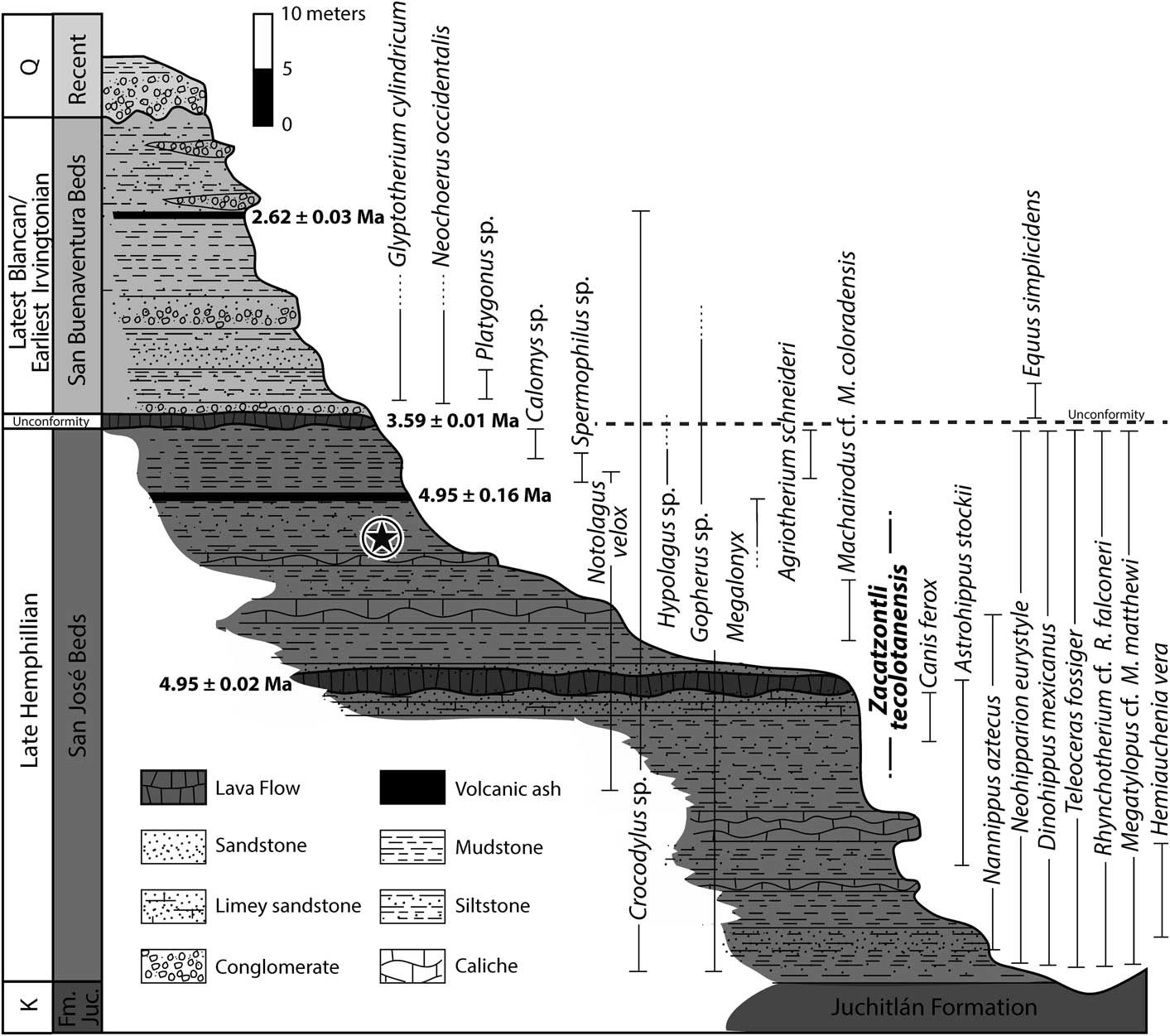
Figure 2 Composite stratigraphic section for the latest Hemphillian to latest Blancan/earliest Irvingtonian in the Tecolotlán Basin and the stratigraphic occurrences of the fauna. The star indicates the level at which the type of Zacatzontli tecolotlanensis n. gen. n. sp. (MPG 1312G) was collected.
The Santa María area
This locality is named for its location near a small ranch called Santa María, at the southeast portion of the basin (20°07'24''N; 104°03'39''W). This general area lies along a complex system of deep arroyos, and the exact fossil site corresponds to the major canyon, situated just south of the ranch. Here, the thickness of the sediments measures 28–30 m. At the base of the section are layers of gravel covered by clays with sands of different sizes. This alternation of clays with sands is only interrupted by a horizon of compacted and characteristically reddish clay. Above this clay is a paleo-channel composed of fine-grained sand from which several rodent molars, along with the two megalonychid molariforms referred to the new taxon described in this work, were found. At the top of the fossiliferous paleo-channel, there are several layers of clays and gravel, and the sequence ends with a series of lacustrine sediments and layers of clay that are 2–6 m thick and show evidence of soil development and roots. Above the whole section there are sandy outcrops cemented by carbonates in which freshwater gastropods are common. It is interesting to note that the cemented sands occur along the entire basin, separating the lower fluvial-lacustrine sediments of the Santa María sequence from the higher San José beds.
The San José beds area
This sequence is well represented at the locality called Jal Teco 20 La Hacienda (20°08'56''N; 104°04'56''W). Access to the locality is by Highway 81 from Tecolotlán. The site is near the ruins of an old mansion called La Hacienda, from which the locality name is derived.
The thickness of this section, measured from the top of the consolidated carbonate sands, is 20–30 m. It consists of layers of clays and sands at the base, and is covered by clayish sediments. It is from this sequence that the jaw of the new megalonychid taxon described in this paper was collected. Stratigraphically, it was found about one meter below a layer of volcanic ash and 12 m above the base of the unit. The tuff from the San José beds produced some grains of sanidine that were dated by 40Ar/39Ar and gave an age of 4.95±0.02 Ma (Kowallis et al., Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017). More recently another analysis of the same layer from a different site was analyzed by the U/Pb method using zircons, which produced a date of 4.85±0.1 Ma for the ash (LA-ICPMS), by Laboratorio de Estudios Isotópicos, Centro de Geociencias, UNAM. The results of these two radiometric assessments are consistent and correspond with the estimated biostratigraphic ages based on the mammalian species present in the San José beds.
Resting on the upper part of the sequence, above an erosional unconformity, there is a more recent unit composed of sands, gravel, and sandy claystones. This part of the section has been named “The San Buenaventura beds.” These sediments are thought to be Blancan in age, and their thickness varies at different sites. The most complete section is at the Jal Teco 7 Las Gravas locality, with at least 30 m of exposed sediments. The basal bed is irregular and its lower contact with the San José beds is not exposed. In general, the lithology of the sequence consists of layers of sandy clay, paleo-channels with gravels, some sandy layers of variable thicknesses, and other strata with sands and many paleo-channels.
Locality Jal Teco 47 Los Pitahayos
In the southeastern part of the basin, near the town of Juchitlán, another late Blancan deposit was described only 300 m away from the Los Corrales ranch, the Jal Teco 47 Los Pitahayos locality (20°05´02”N; 104°05'38''W).
The thickness of the sediments at the site is ~20 m. The fauna includes two South American immigrants, Neochoerus occidentalis and Glyptotherium cylindricum, along with Equus simplicidens Cope, Reference Cope1892, aff. Stockoceros, Platygonus sp., osteoderms and teeth of crocodiles, parts of turtle carapace, and fish remains (operculae). The late Blancan age assigned to this unit was estimated through the analysis of a pyroclastic fall tuff, discovered within the sediments, which was dated using 40Ar/39Ar and gave a date of 2.62±0.03 Ma (Kowallis et al., Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017).
At the bottom of the stratigraphic sequence the sediments that correspond to this part of the section at Santa María are buried by alluvium. The San José beds at this locality are represented by paleosols, clay, and sands that are ~3 m thick. These sediments represent a marsh environment, as indicated by the preservation of tubers and other plant remains. These sediments are covered by layers of consolidated sands that contain gastropods. The mammalian remains were recovered from the top of this sequence and include Megalonyx sp. Jefferson, 1799; Dinohippus mexicanus Lance, Reference Lance1950; Neohipparion eurystyle Matthew, 1909; Teleoceras fossiger (Cope, Reference Cope1878); Rhynchotherium cf. R. falconeri Osborn, Reference Osborn1923; and Hemiauchenia vera Matthew, 1909.
Table 1 Measurements of the mandibles of Zacatzontli tecolotlanensis n. gen. n. sp. (MPG 1312G), Pliometanastes protistus Hirschfeld and Webb, Reference Hirschfeld and Webb1968 (UCMP 97371), and Megalonyx curvidens Matthew, Reference Matthew1924 (FAM 77800); measurements in mm; *approximate length (posterior margin broken); – data not available.

Age and associated fauna
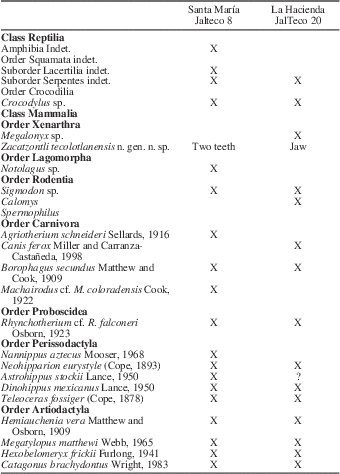
The associated Hemphillian fauna of the Tecolotlán basin is represented in two main localities. In the lower part of the stratigraphic sequence is the Santa María fauna, considered to be late Hemphillian (Hh3), based on the abundance of the equids Nannippus aztecus Mooser, Reference Mooser1968; Neohipparion eurystyle (Cope, Reference Cope1893); and Dinohippus mexicanus Lance, Reference Lance1950; along with limited remains of Astrohippus stockii Lance, Reference Lance1950; Teleoceras fossiger (Cope, Reference Cope1878); and the carnivores Borophagus secundus Matthew and Cook, Reference Matthew and Cook1909; Canis ferox Miller and Carranza-Castañeda, Reference Miller and Carranza-Castaneda1998; and Agriotherium schneideri Sellards, Reference Sellards1916 (Carranza-Castañeda et al., Reference Carranza-Castañeda, Aranda-Gómez, Wang and Iriondo2013). A list of the mammalian fauna is provided in Table 2. Above the Santa María fauna is the La Hacienda fauna, which is also considered to be Hh3 in age because the only difference between the two faunas is the absence of Nannippus aztecus in the latter. A volcanic ash interbedded with the fauna was dated using the 40Ar/39Ar method and yielded an age of 4.95±0.02 Ma (Kowallis et al., Reference Kowallis, Christiansen, Carranza-Castañeda, Miller, Ross and Tingey2017). A new analysis in the same volcanic ash, carried out by the Laboratorio de Estudios Isotópicos del Centro de Geociencias, UNAM, (LA-ICPMS), based on U-Pb in zircons and using laser ablation gave an age of 4.85±0.1 Ma. Based on the fauna, the Santa María fauna correlates with the Rhino layer of Rancho El Ocote and the lower layer of the Coecillos locality in the state of Guanajuato. Both faunas also include Megalonyx (Table 2).
Table 2 Faunal list of the late Hemphillian deposits of Tecolotlán, Jalisco, Mexico.
Systematic paleontology
Order Xenarthra Cope, Reference Cope1889
Suborder Pilosa Flower, Reference Flower1883
Family Megalonychidae Gervais, Reference Gervais1855
Genus Zacatzontli new genus
Remarks
Because Zacatzontli n. gen. is a monospecific genus, all information related to it is given below under the new species.
Zacatzontli tecolotlanensis n. gen. n. sp.
Figure 3 Table 1
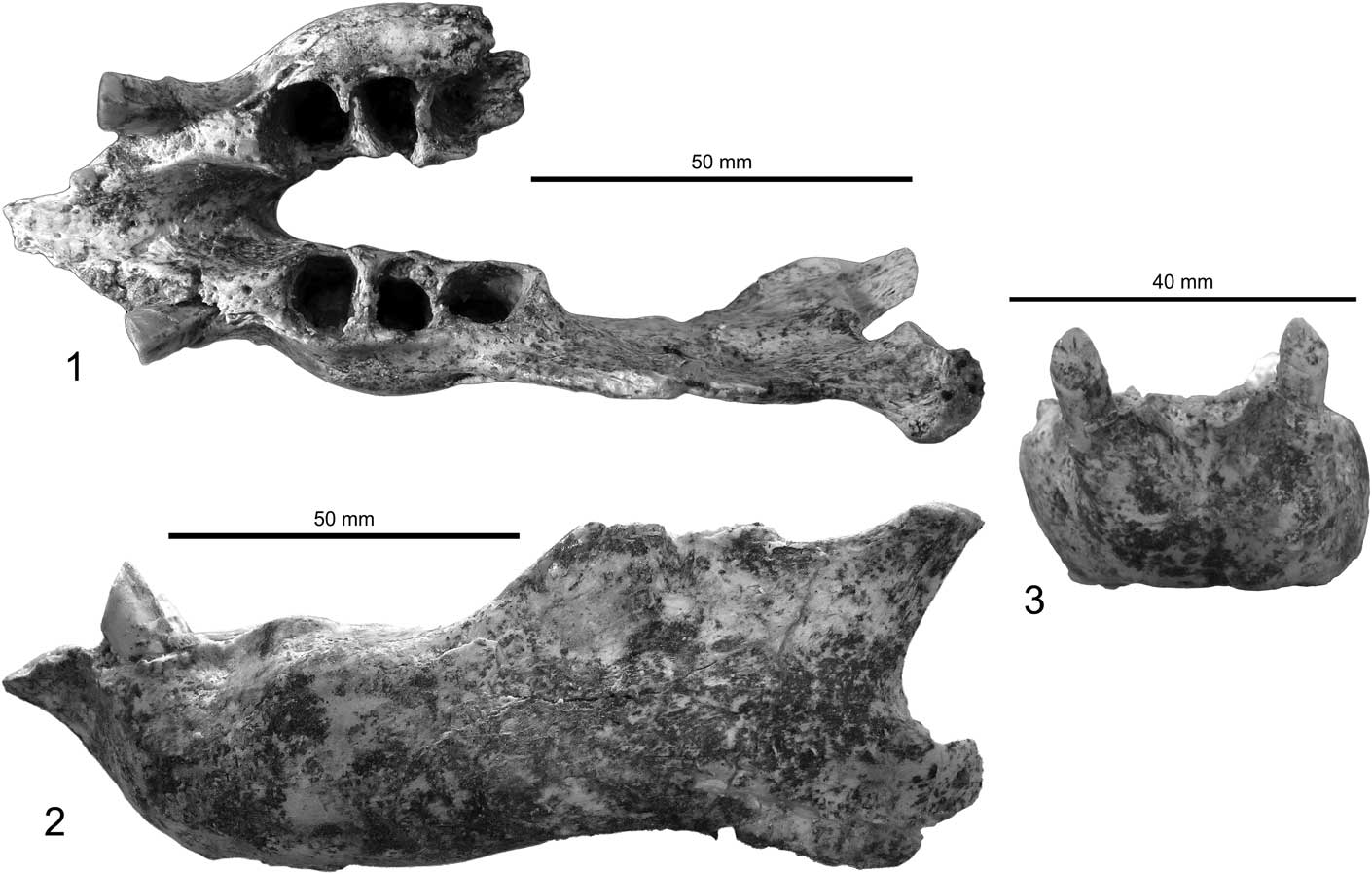
Figure 3 Mandible of Zacatzontli tecolotlanensis n. gen. n. sp. (MPG 1312G): (1) dorsal, (2) left lateral, and (3) anterior views.
Holotype
MPGJ 3126, partial mandible with complete left ramus missing dorsal portion of the coronoid process, posterior margin of the angular process and anterior portion of the right horizontal ramus broken at the midline of the alveolus of the third molariform. Both caniniforms are present, but the molariforms are missing.
Referred specimens
MPGJ 1381, lower left third molariform and MPGJ 3534, lower right first molariform; both specimens from Jal-Teco 8 Santa María Locality.
Etymology
Zacatzontli (Nahuatl) named for the Aztec god of travelers, in reference to the sloth dispersing from South to North America. Specific epithet tecolotlanensis named for Tecolotlán (“place of owls,” Nahuatl) the town and municipality in Jalisco, Mexico near where the specimen was found.
Type locality
Jal Teco 20 La Hacienda, Tecolotlán, Jalisco, Mexico 20°8.44´69”N; 104°4´37.39”W, elevation 1177 m.
Age
Late Miocene, Hemphillian, Hh3 and older than the radiometric date of ash at 4.89 Ma and associated fauna; San José beds.
Diagnosis
Small ground sloth similar in size to the smaller Late Pleistocene megalonychids Acratocnus and Neocnus, from the Caribbean and Eucholoeops and Proschismotherium from the late Miocene of Argentina; lower caniniform triangular in cross section with prominent carina on distal margin of the tooth; predental spout triangular in dorsal view quickly narrowing anteriorly; keel on mandibular symphysis restricted to dorsal half, with small fossa between keel and caniniform in which is located the mental foramen; horizontal ramus below molariforms uniform in anterodorsal width and lacks prominently convex ventral margin; prominent lateral bulge of horizontal ramus opposite first and second molariforms, prominent groove at base of condylar process and anterodorsal margin of angular process.
Description
The specimen consists of a complete left mandible missing only the dorsal portion of the coronoid process, posterior margin of the angular process and the posterior portion of the right horizontal ramus broken at the midline of the alveolar of the third molariform. The caniniforms are present, but the molariforms are missing (Fig. 3). There is some slight breakage along the margins of the predental spout, but this damage does not preclude being able to determine the morphology of the spout.
The lower caniniform has a mesial tilt so that its long axis is at ~45° to the horizontal ramus. It is triangular in cross section with the apex of the triangle on the distal side. The long axis of the tooth slopes slightly anterior so that the occlusal surface has formed at an acute angle to the axis of the tooth, resulting in it being at a right angle to the plane of the tooth row. The occlusal surface of the caniniform faces mesially and is worn at ~45° angle to the axis of the tooth. The mesiolingual and mesiolabial corners of the caniniform are rounded. The distal apex of the tooth has a distinct carina that extends the length of the tooth. The caniniform is slightly displaced labially so its lingual margin is in the same plane as the midline of the molariform series.
The spout is triangular and quickly narrows from its base just anterior to the caniniforms to a point. The triangular shape of the predental spout is more similar to Megalonyx than Pliometanastes. In Zacatzontli n. gen., the anterior margin of the spout is relatively narrower for its width than in Megalonyx. In Pliometanastes, the predental spout is longer, and retains essentially the same width its entire length. In Zacatzontli n. gen., the length of the spout is 30% of the length of the anterior of the caniniform to the distal margin of the m3. This value is 18–20% in Megalonyx curvidens Matthew, Reference Matthew1924 (FAM 77800 from Pliohippus Draw, Nebraska) and 40% in Pliometanastes protistis Hirschfeld and Webb, Reference Hirschfeld and Webb1968 (UCMP 97371 from Siphon Canal, California). The dorsal surface of the spout is concave and continuous with the concave surface of the interior surface of the mandibular symphysis. The posterior margin of the symphysis ends at about the midpoint of the diastema and anterior to the first lower molariform. On the anterior midline of the spout there is a well-defined keel on the dorsal half; ventral to the keel the surface is flat. There is a shallow concavity on either side of the keel in which is located the mental foramen (Fig. 4).
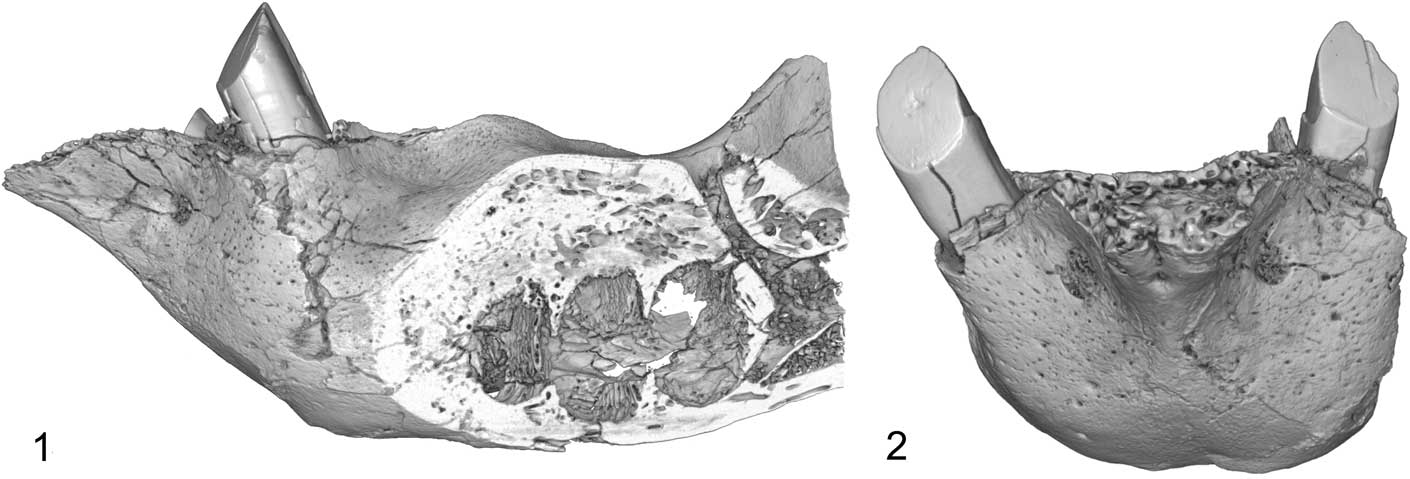
Figure 4 CT scan of mandible of Zacatzontli tecolotlanensis n. gen. n. sp. (MPG 1312G): (1) lateral view through sagittal place of alveoli and showing posterior mandibular canal, and (2) anterior view.
The spout of the mandible in Zacatzontli n. gen. is perhaps one of its most distinctive features when compared to coeval megalonychid sloths in both North and South America. Its short triangular shape is reminiscent of Megalonyx, often considered the most derived spout morphology in the family. In the other Hemphillian and older megalonychids, the predental spout of the mandible is elongated and exceeds the length of the diastema (Fig. 5).
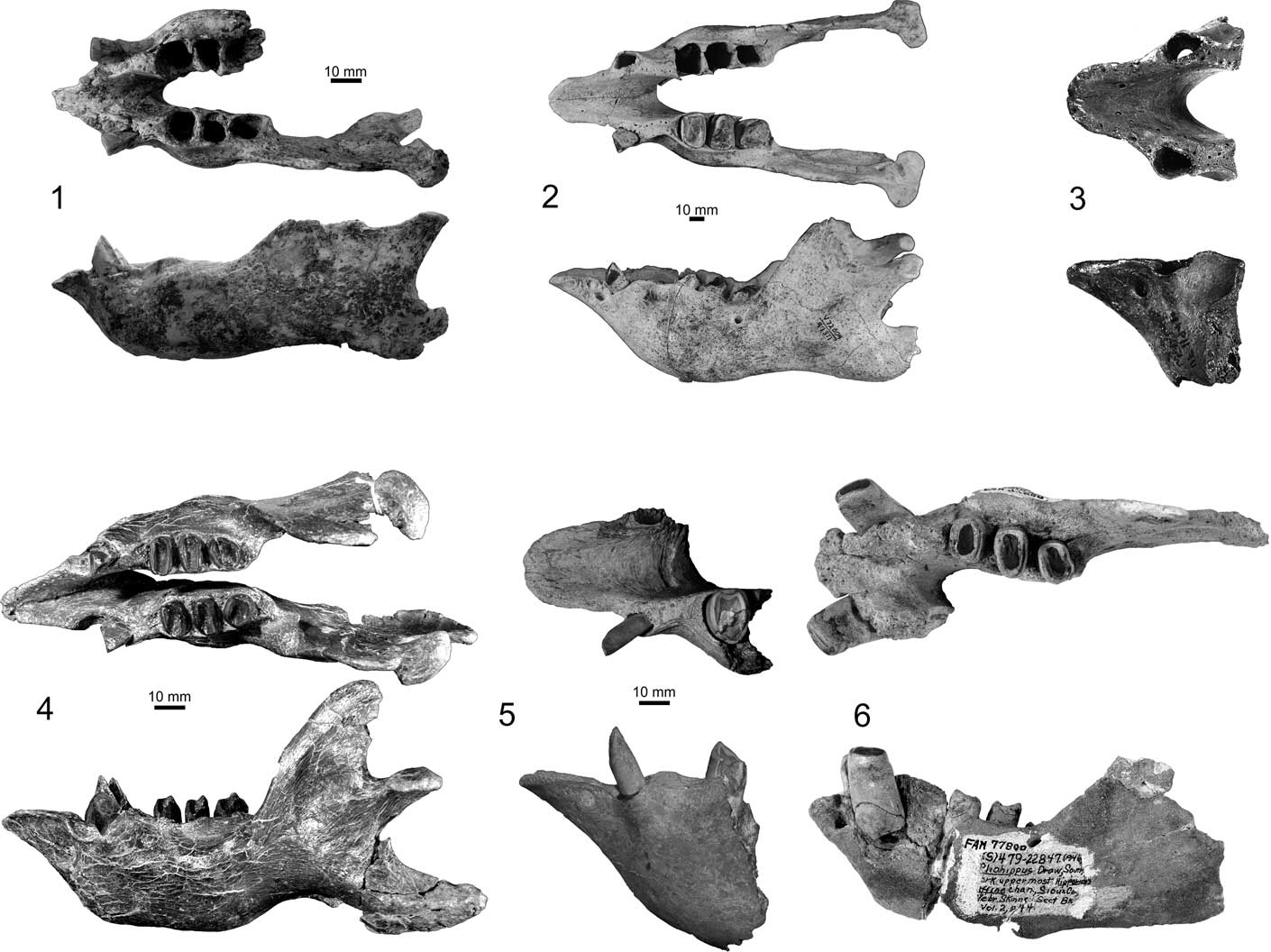
Figure 5 Comparison of anterior jaw morphology of Miocene megalonychid sloths in dorsal and lateral views: (1) Zacatzontli tecolotlanensis n. gen. n. sp. (MPG 1312G); (2) Pliometanastes protistus (UCMP 97371); (3) Pliometanastes protistus (UF 11941); (4) Eucholoeops ingens Ameghino, Reference Ameghino1887 (MPM-PV 3401); (5) Ortotherium laticurvatum Ameghino, Reference Ameghino1885 (MACn PV-13657); (6) Megalonyx curvidens (FAM 77800); (3) and (6) after Hirschfeld and Webb (Reference Hirschfeld and Webb1968); (4) after Bargo et al., Reference Bargo, Toledo and Vizcaíno.2012; (5) after Brandoni, Reference Brandoni2010.
The diastema is relatively long, ~29% of the length of the molariform series. It is relatively thick and the lateral side has the concave buccinator fossa, which extends from the posterior margin of the alveolus of the caniniform to the anterior margin of the alveolus of the first molariform.
Although the lower molariforms have been lost, the shapes of the alveoli permit a determination of their outline and size. The lower molariforms are all roughly the same size and only slightly larger than the caniniform. The horizontal ramus at the level of the molariforms is expanded laterally, a feature also seen in the middle Pleistocene genus Meizonyx (Webb and Perrigo, Reference Webb and Perrigo1985). The ventral margin of the horizontal ramus is not strongly convex and essentially horizontal, so maintains a uniform width from the caniniform to the third molariform and does not become dorsoventrally deep as in Megalonyx and other members of the family. The outline of the alveolus of the lower first molariform is oval with the long axis perpendicular to the long axis of the tooth row. The outline of the alveolus of the second molariform is triangular with rounded vertices and one vertex on the lingual side. The outline of the alveolus of the lower third molariform is slightly ovoid with the axis from mesiolingual to distolabial.
The anterior margin of the ascending ramus has a posterior inclination. The base of the ascending ramus is at the midpoint of the third molariform so the distal half of the tooth is not visible when viewed laterally. The masseteric fossa is shallow and restricted to the coronoid process. The fossa is not subdivided into two distinct areas for the insertion of the zygomaticomandibularis and deep masseter muscles, which is present in some other megalonychids.
The posteroexternal foramen of the mandibular canal is positioned on the lateral side of the horizontal ramus opposite the mesial margin of the lower third molariform and the anterior margin and base of the coronoid process (Fig. 4). Although its position is similar to that of Pliometanastes and Megalonyx, it opens anterodorsally and not laterally, as in the other two genera.
The posterointernal foramen of the mandibular canal is below the dorsal margins of the molariform alveoli and below the midpoint of the coronoid process. It opens into a shallow fossa that extends below the notch between the coronoid and condyloid processes. There is a complimentary convexity on the lateral side of the dentary.
The long axis of the condyle is from anterolateral to posteromedial. The articular surface is horizontal and its posterior margin is rounded in dorsal view.
The posterior margin of the angular process is broken, but enough is preserved to indicate its posterior portion curves slightly medially and the ventral margin has a slight medial curvature. The ventral portion of the lateral surface has a small elongate fossa that extends from the plane at the posterior base of the coronoid process to the posterior edge of the preserved portion of the angular process just above its ventral margin. In the notch between the angular and condylar process there is a small fossa.
Phylogenetic relationships
McDonald et al. (Reference McDonald, Rincón and Gaudin2013) recovered three major clades within the family Megalonychidae; Caribbean (Megalocnus, Parocnus, Acratocnus, and Neocnus), North American (Pliometanastes and Megalonyx), and South American (Megistonyx and Ahytherium). The question is, to which of these clades does Zacatzontli n. gen. belong? Inclusion within either the Pliometanastes-Megalonyx or Caribbean clade would suggest it is derived from the earliest dispersal into North/Central America or the Caribbean, while inclusion in the Megistonyx and Ahytherium clade would suggest perhaps a later dispersal event into North America. Based on the preliminary analysis based on the mandible, Zacatzontli n. gen. clusters with the South American clade and not the North American or Caribbean clades.
Currently it has not been possible to link the earliest sloth taxa in North and Central America with potential ancestral forms or at least sister taxa in South America. While the mylodont Thinobadistes has been linked to Lestodon in a number of taxonomic analyses (e.g., Webb, Reference Webb1989; Gaudin, Reference Gaudin2004), Lestodon occurs considerably later than Thinobadistes. Likewise the genera Pliometanastes, Megalonyx, Meizonyx, and now Zacatzontli n. gen. are currently only known from North America and lack a South American counterpart. Given the Hemphillian age of Pliometanastes and Zacatzontli n. gen., one would expect to find either of these genera in the Chasicoan, Huayquerian, or Montehermosan SALMAs, which cover the same span of time. While Megalonyx first appears in the late Hemphillian, it is currently considered to have evolved in North America from Pliometanastes. A number of South American megalonychid genera are described from this time interval, including Amphiocnus, Megalonychops, and Paranabradys (McDonald and De Iuliis, Reference McDonald and De Iuliis2008), as well as Pliomorphus, Ortotherium, and Mesopotamocnus from the “Conglomerado osífero” of the Ituzaingó Formation, ‘Mesopotamiense’ which has been considered equivalent to both the Chasicoan and Huayquerian (Brandoni, Reference Brandoni2010, Reference Brandoni2014). This would seem to make these taxa all potential candidates to have either participated in the GABI or at least be potential ancestors or the sister taxa to the North American forms.
Because many of these taxa are known only from the types and some are based on parts of the post-cranial skeleton, this limits any extensive or detailed comparisons with the North American megalonychids. While the skull of Pliomorphus is known, no mandibles have been referred to this genus. Given the significantly smaller size of Zacatzontli n. gen. compared to Pliomorphus, we do not consider it likely they are the same taxon. Ortotherium, Paranabradys, and Mesopotamocnus are known from mandibles, although often incomplete, so while the first two genera are included in our analysis of the taxonomic relationships of Zacatzontli n. gen., many characters could not be scored, which further reduces the strength of any interpretations of its relationship to these taxa. Since Meizonyx from the middle Pleistocene of El Salvador is known from the mandible, it has been included in this analysis.
Despite the many megalonychid genera currently described, many are based on fragmentary remains and could not be included in this or other analyses (e.g., Gaudin, Reference Gaudin2004; McDonald et al., Reference McDonald, Rincón and Gaudin2013). Consequently, our analysis here is only intended to provide a first approximation of the possible relationship of Zacatzontli n. gen. to the other megalonychids with which it can be compared (Fig. 6). A fuller analysis will require the recovery of better-preserved and more complete material, particularly the cranium. Given that the analysis is limited to the mandible and is therefore restricted in the number of characters available, we consider the resulting tree to provide a tentative representation of the phylogenetic relationship of Zacatzontli n. gen. and the megalonychid taxa examined, and it should only be considered a first approximation that will require additional testing as more material becomes available.
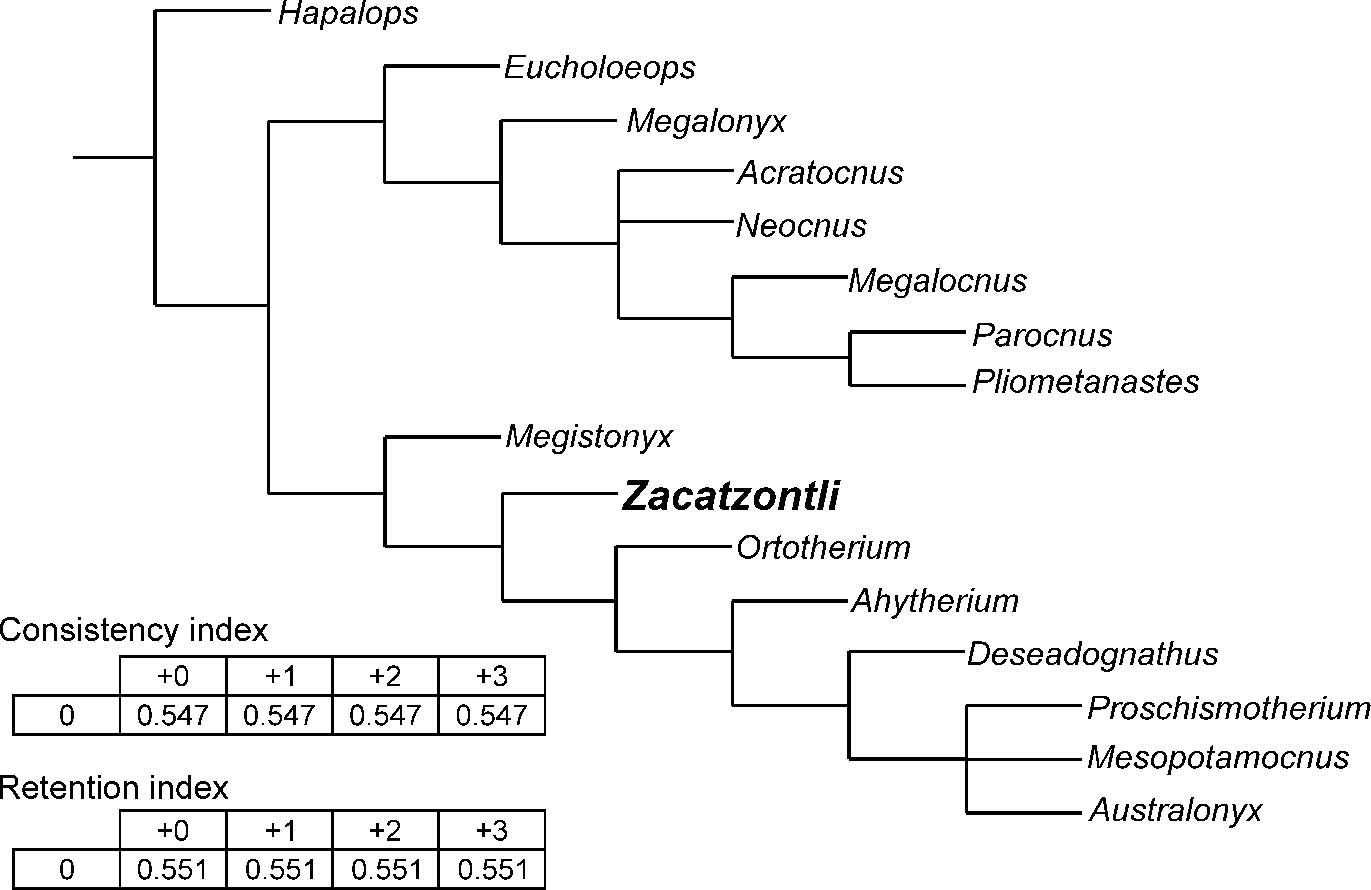
Figure 6 Strict consensus of 4 trees (0 taxa excluded), “Implicit enumeration” based on TNT (Tree New Technologies), Goloboff et al., Reference Goloboff, Farris and Nixon2008; RI=Retention Index, defined to be 0 for parsimony uninformative characters; CI=Consistency Index, CI=1 if there is no homoplasy. Characters used for analysis from Scott, Reference Scott1903–1904; Kraglievich, Reference Kraglievich1923; Simpson, Reference Simpson1932; Matthew and Paula Couto, Reference Matthew and Paula Couto1959; Hooijer, Reference Hooijer1962; Paula Couto, Reference Paula Couto1967; McDonald, Reference McDonald1977; Mayo, Reference Mayo1980; Naples, Reference Naples1982, Reference Naples1987; Patterson et al., Reference Patterson, Segall, Turnbull and Gaudin1992; Gaudin, Reference Gaudin1995; White and MacPhee, Reference White and MacPhee2001; Carlini and Scillato-Yané, Reference Carlini and Scillato-Yané2004; Cartelle et al., Reference Cartelle, De Iuliis and Pujos2008; De Iuliis et al., Reference De Iuliis, Pujos and Cartelle2009, Reference De Iuliis, Cartelle and Pujos2016.
Although this analysis does recover a North American-Caribbean clade and a South American clade (Fig. 6), some of the resulting relationships are quite different from those recovered by Gaudin (Reference Gaudin2004) and McDonald et al. (Reference McDonald, Rincón and Gaudin2013), such as the recovery of Pliometanastes as the sister taxon to Parocnus, and Megistonyx as the sister group to the other South American megalonychids and not to Ahytherium. The position of the oldest megalonychid, Deseadognathus, higher on the tree probably reflects the incompleteness of Deseadognathus, and the unresolved trichotomy of Proschismotherium, Mesopotamocnus, and Australonyx probably reflects the small number of characters in the data set. Despite these limitations, there is a general agreement between our and previous analyses on the broader relationships within the Megalonychidae. The results, therefore, do have heuristic value since we have been able to include genera that have not been included in previous analyses. Given the limited number of taxa represented by mandibles complete enough to obtain a reasonable number of characters, our goal was not to produce a comprehensive taxonomic revision of the family Megalonychidae, but rather to explore at a more basic level the possible relationships of the North and Central American megalonychids to each other and contemporaneous South American genera. Of special interest of course is where Zacatzontli n. gen. fits in these relationships in order to set the stage for future research. Of interest here is whether there was a single lineage of megalonychid sloths that dispersed into Central and North America and subsequently radiated or whether the diversity of megalonychids in Central and North America is the result of multiple distinct lineages of megalonychids dispersing north at different stages of the GABI. Zacatzontli n. gen. is currently known from a single locality dated as Hh3, so occurs later than the earliest well-dated record of Pliometanastes from the Siphon Canal locality in California at 8.19±.16 Ma (Hirschfeld, Reference Hirschfeld1981), which at the moment suggests a separate dispersal. The spout morphology of Zacatzontli n. gen. would appear to make it a better potential ancestor to Megalonyx than Pliometanastes, which has the more primitive elongated spout, but based on this single record it occurs after the earliest records of Megalonyx, which is also present in other similar age sites in Mexico. A second dispersal event of sloths in the later Hemphillian is also suggested by Thinobadistes, which also first occurs in the Hh3 (McDonald and Naples, Reference McDonald and Naples2008).
In our analysis we recovered two major clades within the megalonychids. The North American and Caribbean genera form one group, possibly reflecting their earlier dispersal and possibly a common shared ancestry, and the South American genera, possibly indicating subsequent evolution and diversification after the dispersal that founded the North American-Caribbean clade. The grouping of Zacatzontli n. gen. from the Hemphillian of Mexico with the South American clade rather than with the North American-Caribbean clade suggests that these two major groups of megalonychid sloths had already diverged at this time.
Paleobiogeography
Webb’s (Reference Webb2006) review of the GABI noted that the terrestrial fossil record for mammals in Central and North America unequivocally supports the presence of a significant water barrier between North and South America until ~4–3 Myr. Besides the mammals, evidence for this barrier is also provided by the birds, especially the ecological specialist tropical forest birds (Weir et al., Reference Weir, Bermingham and Schluter2009; Smith and Klicka, Reference Smith and Klicka2010). The age of Zacatzontli tecolotlanensis n. gen. n. sp. suggests that it, like the sloths that dispersed into North America earlier in the Hemphillian, most likely crossed a water barrier in order to enter North America.
McDonald (Reference McDonald2005) noted that of all the mammalian lineages of South American origin that entered North America during the Great American Biotic Interchange (GABI), the sloths were the most successful in terms of taxonomic diversity. The recognition of this new genus and species from the Hemphillian of Mexico increases our knowledge of that overall diversity during the earliest stages of the Great American Biotic Interchange. The discovery of this new sloth also requires a reexamination of McDonald’s (Reference McDonald2005) observation that at any one time there was only one representative of each of the major groups of sloths (megalonychid, nothrothere, megathere, and mylodont) present in North America at each stage of the GABI. This observation was biased by the very robust fossil record of xenarthrans from the temperate part of North America, primarily the United States and northern Mexico, which is in marked contrast to the smaller number of late Cenozoic localities and studies of the fauna from the tropical portion of southern Mexico and Central America. At least for the megalonychidae, we now have more than one representative of the family in the tropical portions of North America.
In addition to Meizonyx salvadorensis Webb and Perrigo, Reference Webb and Perrigo1985 from the middle Pleistocene of El Salvador (Webb and Perrigo, Reference Webb and Perrigo1985), the recognition of a second genus of megalonychid sloth, Zacatzontli n. gen., clearly demonstrates that the taxonomic diversity of fossil xenarthrans (and very likely other groups of South American origin as well) in the tropical portions of North and Central America is much greater than previously thought. This should not be unexpected given that the greatest taxonomic diversity of extant xenarthrans outside of South America today is in Central America and southern Mexico (i.e., the tropics), and includes all three genera of anteaters (Myrmecophaga tridactyla Linnaeus, Reference Linnaeus1758; Tamandua mexicana Saussure, Reference Saussure1860; and Cyclopes didactylus Linnaeus, Reference Linnaeus1758), two armadillos (Cabassous centralis Miller, Reference Miller1899; and Dasypus novemcinctus Linnaeus, Reference Linnaeus1758; the only xenarthran taxon with a range that extends into the United States), and both genera of extant sloths, each represented by a single species (Bradypus variegatus Schinz, Reference Schinz1825 and Choloepus hoffmanni Peters, Reference Peters1858), the “camp followers” of McDonald (Reference McDonald2005).
Webb (Reference Webb2006) noted that while geographically southern Mexico and Central America are geologically tied to North America, having been widely and deeply separated from northern South America by the Bolivar Trough, today fauna and flora of this region are more similar to Neotropical South America—his Central American Paradox. As described by Webb (Reference Webb2006), Central America was conquered by the tropical fauna of South America, which is clearly indicated by the diversity of xenarthrans in this region. Because the majority of these taxa are restricted to tropical rainforest habitat, the northernmost limit for their distribution is at about the same latitude (±16°N) (Fig. 7). As discussed by McDonald (Reference McDonald2005), the variety of habitats in Central and North America acted as “nested sieves” that restricted the northern dispersal of xenarthrans, and it is clear that the majority of taxa dispersing out of South America were adapted to a tropical habitat and hence restricted to how far north their range could extend. While the sloths (Pliometanastes, Megalonyx, Thinobadistes, Glossotherium/Paramylodon, Nothrotheriops, and Eremotherium) and the cingulates (Glyptotherium, Holmesina, Pachyarmatherium, Dasypus bellus Simpson, Reference Simpson1929, and to a lesser extent Pampatherium) extended their ranges into more northern temperate environments for geologically extended periods of time, it is becoming clear that there exists at least as great and possibly greater amount of currently undocumented xenarthran diversity in the tropical part of North America, which is only now being discovered.
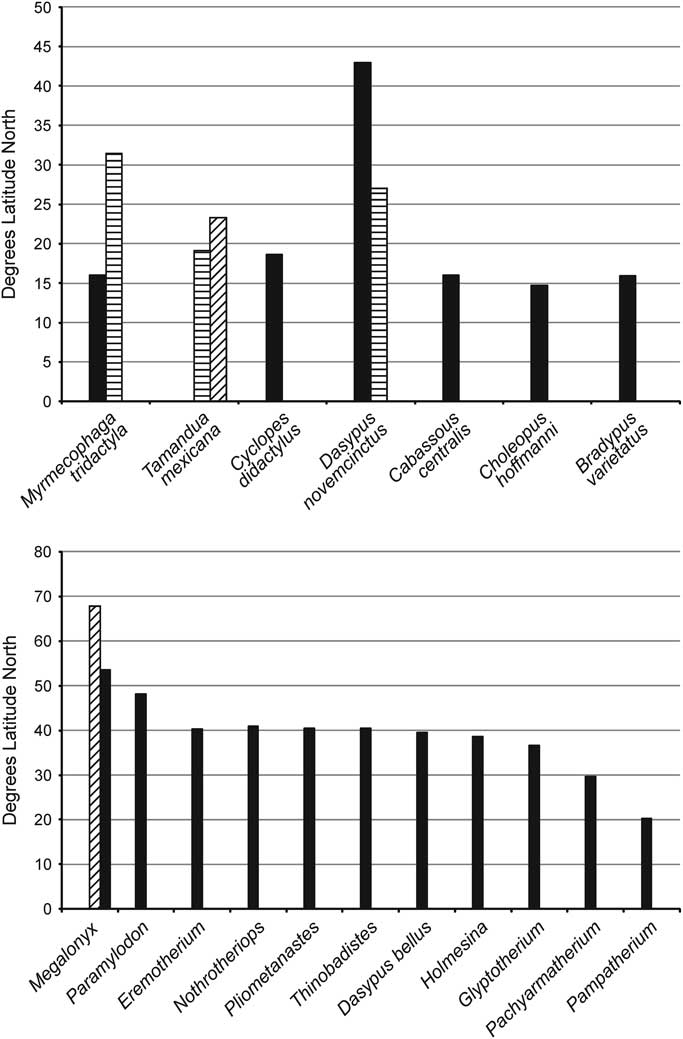
Figure 7 Comparison of maximum latitude of extant and fossil North American xenarthrans: (1) extant taxa, (2) extinct taxa. The striped bar for Myrmecophaga is the Irvingtonian range extension, the two bars for Tamandua show the differences on the Pacific and Gulf coasts, the two bars for Dasypus show the differences on the Pacific and Gulf coasts, and the striped bar for Megalonyx shows its Sangamonian range extension.
Fluctuating climatic conditions through the Pliocene and Pleistocene did permit short-term northerly range expansions of some of the tropical taxa, such as the giant anteater, Myrmecophaga tridactyla, in the Middle Pleistocene (Irvingtonian) El Golfo fauna at 31.5°N (Shaw and McDonald, Reference Shaw and McDonald1987), or even temperate taxa such as Megalonyx expanding its range as far north as the Yukon during the Sangamonian interglacial (McDonald et al., Reference McDonald, Harington and De Iuliis2000).
Today, the Neotropics support an extremely large diversity of living mammals with ~1500 recognized species currently known, representing ~30% of the total world mammal diversity. This biodiversity includes numerous endemic groups such as marsupials, xenarthrans (sloths, armadillos, and anteaters), caviomorph rodents, platyrrhine monkeys, and phyllostomid bats (Patterson and Costa, Reference Patterson and Costa2012). The existence of the variety of biomes present in the Neotropics (lowland rainforest, savannas, mountain forest, scrublands, and deserts) provides a partitioned environment that enhances the overall species richness (Tews et al., Reference Tews, Brose, Grimm, Tielbörger, Wichmann, Schwager and Jeltsch2004) along with the latitudinal gradient diversity (Jablonski et al., Reference Jablonski, Belanger, Berke, Huang, Krug, Roy, Tomasovych and Valentine2013).
Croft (Reference Croft2007) used the Simpson Index of faunal similarity to compare the middle Miocene Quebrada Honda Fauna from mid-latitudes in Bolivia with the slightly older high-latitude fauna of Collón-Curá Argentina and noted that it was more similar to the older fauna than to the contemporaneous low-latitude fauna of La Venta in Colombia. He suggested isolating mechanisms existed between the low and middle latitudes during the early and/or middle Miocene. Carrillo et al. (Reference Carrillo, Forasiepi, Jaramillo and Sánchez-Villagra2015), using dissimilarity analysis, showed that distinct tropical and temperate faunal assemblages existed in South America during the middle Miocene (Colloncuran–Laventan [≈ Langhian–Serravallian]) and late Miocene (Huayquerian–Montehermosan [≈ Tortonian–Piacenzian]). Their analysis of first appearances of taxa in the faunas examined showed that across time and latitude the faunistic movements related to GABI began during the late Miocene (~10 Ma) with the oldest records found at higher latitudes. The number of FAD remained relatively low until 4–5 Myr when FAD starts to increase, peaking during the Pleistocene. They concluded that the study of paleodiversity patterns and paleobiogeography in the Americas is currently biased by the samples available (Carrillo et al., Reference Carrillo, Forasiepi, Jaramillo and Sánchez-Villagra2015).
Extrapolating from the possibility that there may exist a diverse number of currently unknown extinct sloth and other xenarthran taxa in the tropical portions of South America brings us full circle to the issue that many of the North American sloth taxa, such as Pliometanastes, Zacatzontli n. gen., and Thinobadistes, are not present in South America from similar-aged faunas, or at least are not known from the well-documented similar-aged faunas of Argentina and the more temperate parts of South America.
Past studies have focused on the dispersal of taxa between North and South America in both directions following the formation of the land bridge by ~3 Ma, as correlated with the expansion of savannas and grasslands in the Neotropics during glacial periods (Webb, Reference Webb1991, Reference Webb2006; Leigh et al., Reference Leigh, O’Dea and Vermeij2014). It appears that North American taxa seem to have been more successful utilizing more temperate biomes, whereas, in contrast, the South American taxa dominated in the tropics (Webb, Reference Webb1991, Reference Webb2006; Leigh et al., Reference Leigh, O’Dea and Vermeij2014). Therefore, attempting to derive the North American sloth taxa from taxa from the temperate part of South America should be reconsidered. Rather, it may be that at least some of the North American sloth taxa are instead derived from an ancestral stock that lived in the northern, and thus tropical, part of South America, which is an area that is greatly underrepresented by faunas in general as well as those that date to the beginning of the GABI. Gaudin and Croft (Reference Gaudin and Croft2015) attributed the rarity of early xenarthran remains not only to the general scarcity of fossil localities from tropical latitudes in South America, but also to low population densities associated with myrmecophagy and the lack of durable, enamel-covered teeth, which have been the basis for many of the described mammalian taxa from this region. The sediments in many of the known sites in tropical South America are marine-fluvial in origin, and therefore represent significantly different environments than inland sites in the temperate latitudes (personal communication, D. Croft, 2017). The large number of early records of sloths in Hemphillian faunas in North America is an artifact of more fieldwork in this region, which is a mirror image of what is seen in temperate South America. With an increase in the recovery of sites in both tropical Central America (Laurito and Valerio, Reference Laurito and Valerio2012) and South America (Rincon et al., Reference Rincón, Solórzano, Macsotay, McDonald and Núñez-Flores2016), it may be just a matter of time before Zacatzontli n. gen. and some of the other “endemic” North American sloth taxa are found in the tropical part of South America and a fuller understanding of xenarthran diversity and the participation of this group during the early stages of the GABI is possible.
Conclusions
The discovery of a new genus and species of megalonychid sloth, Zacatzontli tecolotlanensis, from the late Hemphillian of Jalisco, Mexico increases the diversity of taxa of South American origin that participated in the GABI. Zacatzontli n. gen. in Mexico and another megalonychid sloth, Meizonyx, in El Salvador clearly show there is a greater diversity of taxa of South American origin in the northern Neotropics than had been previously suspected. This reflects the general paucity of Cenozoic fossil sites from the tropical lower latitudes in North America and that the majority of our knowledge of the diversity of taxa that participated in the GABI and the timing of their dispersal into North America has been based on faunas from higher latitudes. The presence of these sloths raises interesting questions in terms of the evolutionary history of the megalonychid sloths and whether their appearance in North America reflects multiple separate dispersal events or a smaller number of dispersals with the subsequent evolution of these taxa occurring in the tropical portions of North America. The presence of these taxa only in North America and their absence in South America suggest this may be the case, but alternatively could simply reflect that, as in North America, there are fewer sites from the late Cenozoic in the tropical parts of northern South America. These questions cannot be resolved until additional fieldwork in the adjoining tropical regions of South and North America provides us with a more robust fossil record.
Acknowledgments
Our thanks to the Centro de Geociencias of the Universidad Nacional Autónoma de México for the support and logistics to carry out the fieldwork and preparation of the fossil material. These were possible with the economic support of the project PAPIIT IN102817. We thank W.E. Miller of Brigham Young University for his participation during the research on the sedimentary basins of central Mexico, B. Kowallis from BYU for description of the geology and stratigraphy and radiometric ages of the Tecolotlán Basin. We thank J. Silva Corona for the figures and photographs in this work, H. Troncoso for the preparation of the fossils collected, C. Luis Espinosa for his criticism of part of the manuscript, and C. Ortega (LA-ICPMS), Centro de Geociencias for the second analysis of ash. The Center for Field Research, and especially Earth Watch volunteers, are thanked for their support of the Fossils of the Sierra Madre project, and their invaluable help collecting fossils during this project. We thank P. Holroyd, UCMP, for providing pictures of the Pliometanastes from the Siphon Canal Locality and L. Fowler for providing the measurements of this specimen. S. Bargo, D. Brandoni, and S. Hirschfeld generously provided images of specimens from their publications. We thank our reviewers, D. Croft and G. DeIuliis, for their comments, which improved the quality of the paper.













