Introduction
Flagship species are those used for broader conservation marketing campaigns and are chosen based on the possession of one or more traits that appeal to the targeted audience (Verissimo et al. Reference Verissimo, MacMillan and Smith2011, Veríssimo et al. Reference Veríssimo, Pongiluppi, Santos, Develey, Fraser, Smith and MacMilan2014). Among the attractive traits are the following: sharing their space with other species (or background species), having cultural significance, and playing a role in ecological processes (Andelman and Fagan Reference Andelman and Fagan2000, Jepson and Barua Reference Jepson and Barua2015). For national and international conservation organisations, flagship species are used as emblems to help in fundraising. For example, the giant panda is an insignia of the World Wildlife Fund for Nature, just as the African elephant for the African Wildlife Foundation and for Save the Elephants (Clucas et al. Reference Clucas, McHugh and Caro2008). On the local scale, flagship species can be used to establish small reserves (e.g., El Rosario Monarch Butterfly Sanctuary, in the state of Michoacán, in Mexico, or La Paz Bay whale shark refuge, in the state of Baja California, Mexico). Thus, flagship species can be employed for conservation awareness and for protection of species and ecosystems.
As a biodiversity conservation tool, flagship species can symbolise nature benefits (e.g., sea turtles for the Wider Caribbean Region; Eckert and Hemphill Reference Eckert and Hemphill2005) and the history of ecosystems (e.g., monitor lizards in the Philippines; Welton et al. Reference Welton, Siler, Bennett, Diesmos, Duya, Dugay, Rico, Van Weerd and Brown2010), in addition to extinction threat concerns. Therefore, species richness, ecological functions, and evolutionary history of other species that coincide in space and time with a flagship species should be considered. The use of functional diversity metrics can provide information about the productivity and stability of ecosystems (Tilman Reference Tilman2001, Mason et al. Reference Mason, Mouillot, Lee and Wilson2005). Furthermore, phylogenetic diversity metrics are useful for understanding the evolutionary history of species assemblages, as well as the processes that formed them (Winter et al. Reference Winter, Devictor and Schweiger2013). Both biological diversity metrics can be useful in defining priority conservation areas and conservation strategies, which are urgently needed for vulnerable ecosystems (Dalerum Reference Dalerum2013).
Worldwide, riparian habitats diminished by 35% from 1970 to 2015, and Latin America has been one of the most affected regions with a loss of 59% (Darrah et al. Reference Darrah, Shennan-Farpón, Loh, Davidson, Finlayson, Gardner and Walpole2019). Riparian habitats provide several ecosystem services, as well as resources for numerous faunal species (Brouwer et al. Reference Brouwer, Tesfaye and Pauw2011, McClure et al. Reference McClure, Korte, Heath and Barber2015, Land et al. Reference Land, Granéli, Grimvall, Hoffmann, Mitsch, Tonderski and Verhoeven2016, Mokondoko et al. Reference Mokondoko, Manson and Pérez-Maqueo2016, Suazo-Ortuño et al. Reference Suazo-Ortuño, Alvarado-Díaz and Martínez-Ramos2011). For amphibians, which are the most threatened vertebrate group, riparian habitats are important as they frequently represent a key habitat for the completion of their life cycles (Becker et al. Reference Becker, Fonseca, Haddad, Batista and Prado2007, Becker et al. Reference Becker, Fonseca, Haddad and Prado2010). Moreover, riparian habitats provide critical food and reproductive resources for amphibians and offer protection from desiccation in modified landscapes (Gray and Smith Reference Gray and Smith2005, Peterman and Semlitsch Reference Peterman and Semlitsch2014, Boissinot et al. Reference Boissinot, Grillet, Besnard and Lourdais2015).
In the state of Michoacán, Mexico, around 40% of the original vegetation cover has been replaced by cropland and cattle pastures (Instituto Nacional de Estadística y Geografía (México) 2017). Northeastern Michoacán is a recognised endemism and diversity centre for amphibians because of environmental conditions associated with altitudinal gradients and the conjunction of two physiographic provinces: the Trans-Mexican Volcanic Belt and the Sierra Madre del Sur (Flores-Villela and Goyenechea Reference Flores-Villela, Goyenechea, Morrone and Llorente Bousquets2003, Urbina-Cardona and Flores-Villela Reference Urbina-Cardona and Flores-Villela2010). In northeastern Michoacán, the proportion of cropland in the landscape influences the distribution of amphibian species more than other types of vegetation cover (i.e., mature and secondary forest, pastures) or altitudinal condition (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022). Thus, defining conservation strategies is urgently needed for protecting amphibian assemblages and guaranteeing both their ecosystem functions and their evolutionary history.
The endemic salamander Ambystoma ordinarium is a species at risk (IUCN 2023) that is found in northeastern Michoacán. This salamander can inhabit mature riparian forest and streams surrounded by up to 50% of cropland (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido and Munguía-Steyer2021, Piñon-Flores et al. Reference Piñon-Flores, Suazo-Ortuño, Ramírez-Herrejón, Moncayo-Estrada and del-Val2021). Furthermore, riparian areas inhabited by A. ordinarium showed higher numbers of amphibian species at risk (i.e., high values from the Environmental Vulnerability Index; See Table 1 from Methods Section) than areas without this salamander (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022). In addition to extinction threat concerns, we propose that A. ordinarium can be considered as a flagship species in northeastern Michoacan since some other Ambystoma species are associated to the plight of conserving threatened aquatic ecosystems (e.g., Ambystoma mexicanum or A. dumerilli; Bride et al. Reference Bride, Griffiths, Meléndez-Herrada and McKay2008; Velarde-Mendoza Reference Velarde-Mendoza2012). These salamanders have an important role in local culture and traditional medicine, in Mexico (Díaz-García et al. Reference Díaz-García, Oropeza-Sánchez and Aguilar-López2019). Also internationally, Ambystoma salamanders are recognised by medical science as a result of their regeneration capacity (Tanaka Reference Tanaka2003).
Table 1. Amphibian species detected in riparian habitats from northeastern Michoacán and their morphological traits. Those species at risk (with higher EVI values) are presented in a shaded color. We show the following: type of microhabitat most frequently used (semi-aquatic = SemiAqua; terrestrial = Terr; arboreal = Tree; and aquatic = Aqua); type of breathing (pulmonary = Pulm; cutaneous = Cut; and gill = Gill); microhabitat used for spawning (Lotic, Lentic, Tree, Rock or Land); the presence of free aquatic larvae, presence of interdigital webbing, presence of toe pads, and snout-vent length. Information about egg place was taken from references at the bottom of the table.
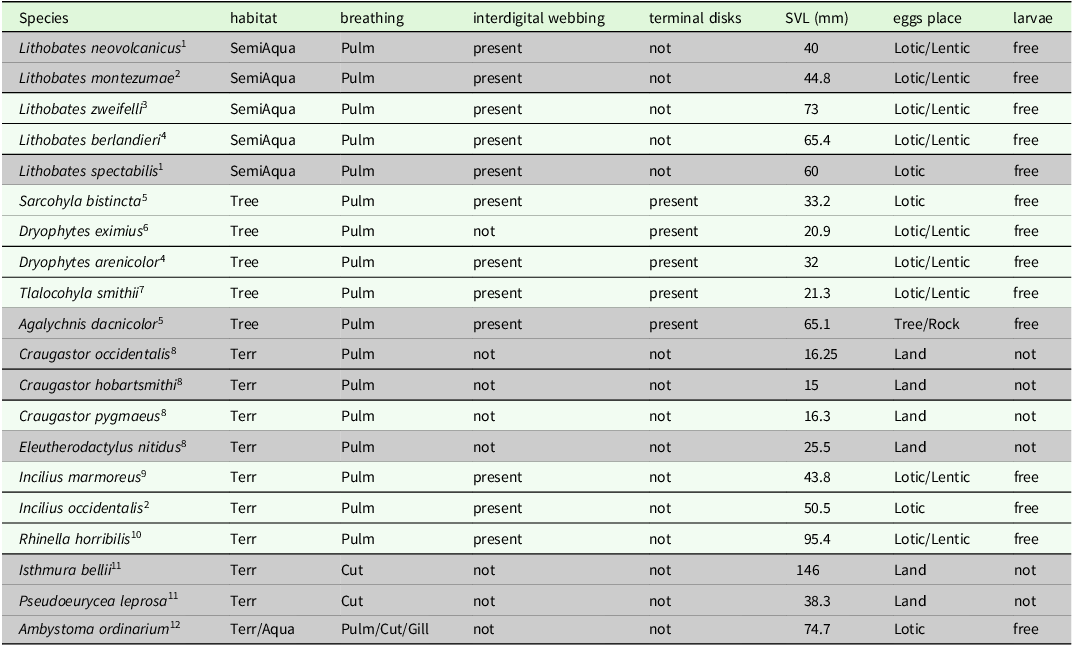
(1) Hillis and Frost (Reference Hillis and Frost1985); (2) IUCN (2023); (3) Canseco-Márquez and Gutiérrez-Mayén (Reference Canseco-Márquez and Gutiérrez-Mayén2010); (4) Dodd (Reference Dodd2013); (5) Duellman (Reference Duellman2001); (6) Reyna-Bustos et al. (Reference Reyna-Bustos, Ahumada-Carrillo and Vázquez-Huizar2007); (7) Álvarez-Grzybowska (Reference Álvarez-Grzybowska2016); (8) Duellman and Trueb (Reference Duellman and Trueb1994); (9) Duellman (Reference Duellman1961); (10) AmphibiaWeb (2023); (11) Stuart et al. (Reference Stuart, Hoffmann, Chanson, Cox, Berridge, Ramani and Young2008); (12) Anderson and Worthington (Reference Anderson and Worthington1971).
Besides A. ordinarium, we have estimated high occupancy probabilities for some amphibians in riparian surrounded by up to 50% of cropland, but these species present different traits (e.g., microhabitat preference; Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022). Because ecological traits are frequently result of phylogenetic conservatism (e.g., Kozak and Wiens Reference Kozak and Wiens2010, Sato et al. Reference Sato, Kusumoto, Şekercioğlu, Kubota and Murakami2020), modified riparian habitats could maintain both a high functional and high phylogenetic amphibian diversity. Since A. ordinarium co-occurred with disturbance-sensitive and disturbance-tolerant species, we predict that the protection of this salamander could provide further protection to amphibian assemblages with high functional and phylogenetic diversity. The objective of the present study was to evaluate amphibian functional and phylogenetic diversity between riparian habitats with and without the incidence of A. ordinarium and therefore to potentially support the use of this species as a flagship species for amphibian conservation.
Materials and methods
Potential flagship species
The Michoacan stream salamander (Ambystoma ordinarium) is an endangered member of the Ambystomatidae family. This salamander family includes species which remain with juvenile morphology all their lives and are restricted to aquatic habitats (paedomorphosis), but there are some cases where this feature is facultative, such as A. ordinarium (Vitt and Caldwell Reference Vitt and Caldwell2013). Because of this attribute, A. ordinarium can go through a metamorphosis to reach terrestrial habitats and avoid some detrimental aquatic conditions (e.g., shallow pools or low oxygen levels; Denoël and Ficetola Reference Denoël and Ficetola2014). Currently, this salamander family presents population declines strongly associated with landscape modifications because of human activities (e.g., urbanisation and land-use change; AmphibiaWeb 2023). Distribution of A. ordinarium is restricted to riparian habitats above 1900 m.a.s.l in northeastern Michoacán (Anderson and Worthington Reference Anderson and Worthington1971, Soto-Rojas et al. Reference Soto-Rojas, Suazo-Ortuño, Montoya Laos and Alvarado-Díaz2017), and its total occupation range is less than 500 km2 (IUCN 2023).
Study area
The study area was delimited by combining the projected area of occupancy of Ambystoma ordinarium (IUCN SSC Amphibian Specialist Group 2015) and its potential distribution area in northeastern Michoacán (Escalera-Vázquez et al. Reference Escalera-Vázquez, Hernández-Guzmán, Soto-Rojas and Suazo-Ortuño2018; Figure 1). The original vegetation of the study area consists of pine forest, pine-oak forest, cloud mountain forest, and deciduous forest (Rzedowski Reference Rzedowski2006). The elevation ranges from 700 to 3000 m.a.s.l, and the mean annual temperature varies from 10 to 26°C. Furthermore, the annual rainfall ranges from 600 to 1500 mm (Cuervo-Robayo et al. Reference Cuervo-Robayo, Téllez-Valdés, Gómez-Albores, Venegas-Barrera, Manjarrez and Martínez-Meyer2014a, Reference Cuervo-Robayo, Téllez-Valdés, Gómez-Albores, Venegas-Barrera, Manjarrez and Martínez-Meyer2014b).
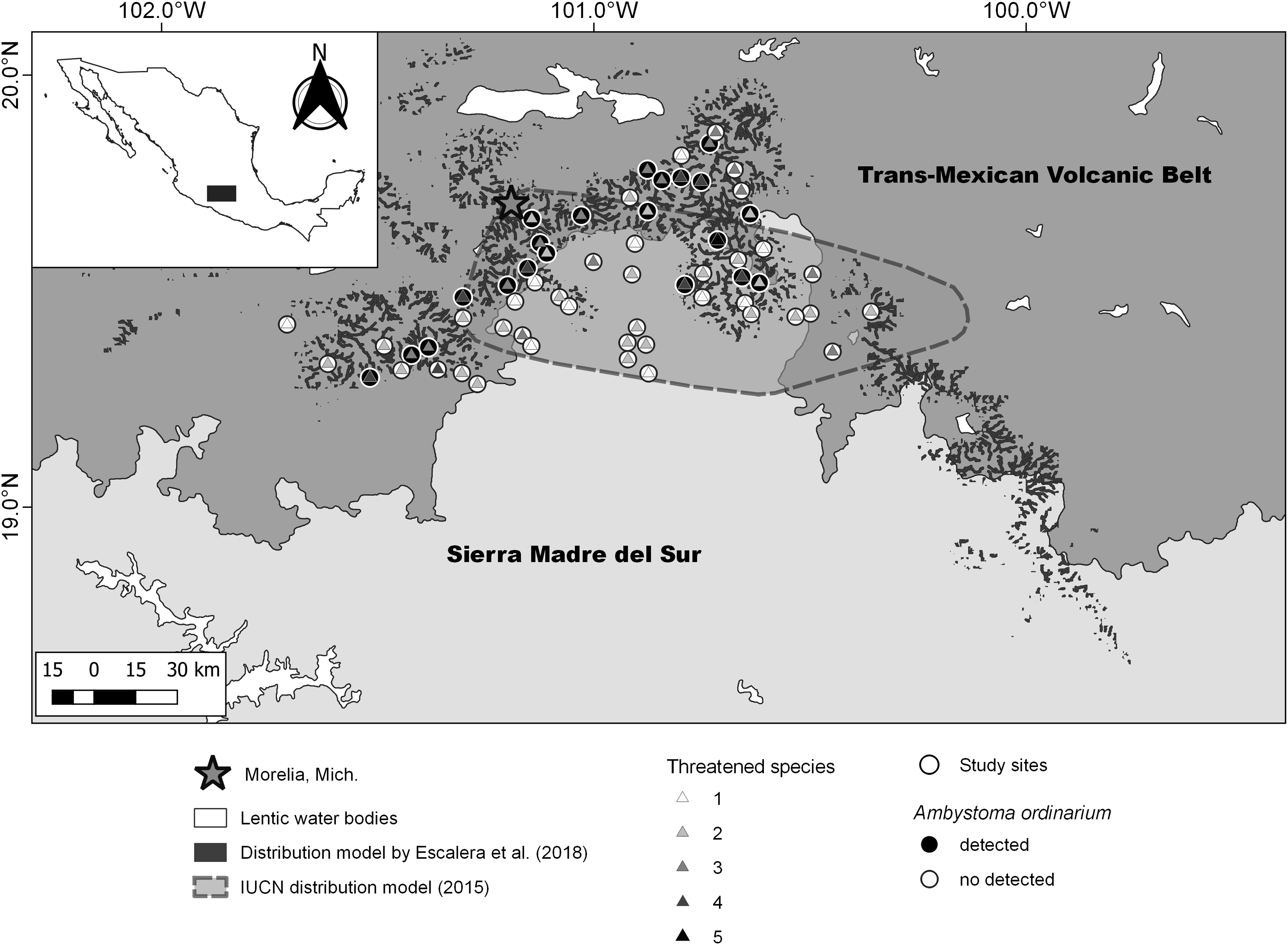
Figure 1. Estimated values of threatened species, geographic distribution model of Ambystoma ordinarium, and sampling unit with detection in northeastern Michoacán, Mexico.
Data collection
To record the amphibian species inhabiting the study area, we selected 60 stream sections and the adjacent riparian zones. Considering the average dispersal capacity of amphibians (Rittenhouse and Semlitsch Reference Rittenhouse and Semlitsch2007), the selected stream sections with independent flow were at least 3 km apart to ensure their spatial independence. The extension of the study area was approximately 5,000 km2 (see Figure 1). In each stream section, a fixed 50 x 25-m plot that included the riparian zone was established. Each plot was considered a sampling unit. To represent a gradient of land-use change, we selected stream sections with different proportions of mature forest. From February 2018 to October 2019, we conducted 12 sampling events in each sampling unit. The search for amphibians was only diurnal, between 09.00 and 18.00 h, because of logistical problems (e.g., insecure conditions). The amphibian search was performed by inspecting terrestrial microhabitats (in tree hollows, under logs and rocks, in leaf litter and on riverbanks) and aquatic microhabitats (at the bottom of the stream channel and under roots and rocks). This sampling design allowed us to record both active diurnal and inactive nocturnal species. A 30-cm diameter hand net was used for capturing the amphibians. In each unit, the sampling effort was standardised to 40 person-minutes per sampling event. We only recorded detections of juveniles and adults; all specimens collected in the field were identified to species level. Except for those species at risk, one individual of each species was deposited in the herpetological collection of the Universidad Michoacana de San Nicolás de Hidalgo.
Data analysis
Detection data (e.g., abundance and presence) are strongly influenced by sampling design and environmental conditions; thus, these records can represent an unsuitable approximation of species contribution to assemblages. We used species detection histories to calculate the average occupation state of each amphibian species in the sampling units, then we estimated functional and phylogenetic traits. Values of the occupation state of the species j in each of the sampling units i (z ij ) were obtained from the most likely model of six multispecies occupancy models (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022). In the selected model, we considered the linear and quadratic effect of the proportion of cropland to explain occupancy probability, as well as humidity, to estimate the probability of detection of 20 amphibian species (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022). To perform the statistical analyses, we considered the occupation state values z ij ≥ 0.194 of each species j in each sampling unit i. This threshold value was defined to avoid overestimations in diversity metrics.
Functional diversity
To estimate functional diversity, we considered species morphological traits that may be associated with microhabitat use, as well as reproductive strategy and biomass (Díaz-García et al. Reference Díaz-García, Pineda, López-Barrera and Moreno2017, Alvarez-Grzybowska et al. Reference Alvarez-Grzybowska, Urbina-Cardona, Córdova-Tapia and García2020). For each species sampled, we recorded the following traits in the field: most frequently used microhabitat, respiration type, the presence of interdigital webbing, the presence of toe pads, snout-vent length (SVL) in millimetres, microhabitat used for spawning, and the presence or absence of free aquatic larvae (Table 1). We reviewed specialised literature to obtain information about reproductive traits (see Table 1). To estimate functional diversity in each sampling unit, we used four complementary metrics: functional richness (FRich), functional evenness (FEve), functional divergence (FDiv), and functional dispersion (FDis) (Villéger et al. Reference Villéger, Mason and Mouillot2008, Laliberté and Legendre Reference Laliberté and Legendre2010). FRich measures the minimum convex hull occupied by species in an assemblage in the functional space defined by species traits, and FEve expresses the abundance regularity of the species traits in the functional space of assemblages. Furthermore, FDis is the average distance of species with respect to the centroid in the trait space and measures the spread of species around a theoretical functionally average species, while FDiv estimates the degree to which the abundance distribution maximises the dispersion of functional traits of assemblages and is interpreted as a measure of functional similarity between dominant species (Laliberté and Legendre Reference Laliberté and Legendre2010, Mouchet et al. Reference Mouchet, Villéger, Mason and Mouillot2010, Pla et al. Reference Pla, Casanoves and Di Rienzo2011).
We weighted Feve, FDiv, and FDis according to occupancy state (z ij ) values of each species by sampling unit. Weighting these metrics with the occupancy state does not imply that z ij values are a substitute for species abundance. By employing z ij values, however, we assume that the occupancy probability of species in a local assemblage is related to the probability that its function is realised in this assemblage (Bender et al. Reference Bender, Kissling, Böhning-Gaese, Hensen, Kühn, Nowak, Töpfer, Wiegand, Dehling and Schleuning2019). Therefore, species with a lower occupancy state in an assemblage will affect the metrics less than those with higher values of z ij.
To remove the influence of species richness on functional diversity metrics, we estimated their standardised values. First, we generated a random distribution under the null hypothesis of no association between A. ordinarium distribution area and traits of amphibian assemblages. Our null model developed from 1000 permutations of site × species occupancy state (z ij ) matrix, employing the ‘independent swap’ algorithm (Gotelli Reference Gotelli2000; Henriques et al. Reference Henriques, Rigal, Borges, Ah-Peng and Gabriel2017). This algorithm randomises species occurrence values maintaining the total of species richness or the species frequency observed of each sampling unit. Then, we estimated the standardised values (SES), by the following formula: SES = (Obs-µ null)/ σ null, where Obs is each functional metric estimated initially (FRich, FEve, FDis, or FDiv), µ null and σ null are the mean functional metric from simulated communities and their standard deviation, correspondingly (Swenson Reference Swenson2014). The standardised values allow quantifying when functional diversity metrics are lower (negative values), higher (positive values), or not differed (zero values) from the average random expected. The estimations were conducted using R (version 4.0, R Core Team, 2020), as well as the statistical packages FD (Laliberté et al. Reference Laliberté, Legendre and Shipley2014) and picante (Kembel et al. Reference Kembel, Cowan, Helmus, Cornwell, Morlon, Ackerly, Blomberg and Webb2010).
Phylogenetic diversity
To evaluate the phylogenetic diversity, we built a regional phylogeny that included the 20 species recorded in our study (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Santos, Pyron, Arroyo-Rodríguez, Urbina-Cardona, Martínez-Ramos, Parra-Olea and Reynoso2019) by pruning the globally calibrated phylogeny of 2,800 amphibian species of Pyron and Wiens (Reference Pyron and Wiens2011). For each sampling unit, we estimated the mean pairwise distance (MPD), mean distance to the nearest taxon (MNTD), and standardised values of both metrics (Swenson Reference Swenson2014; Tucker et al. Reference Tucker, Cadotte, Carvalho, Jonathan Davies, Ferrier, Fritz, Grenyer, Helmus, Jin, Mooers, Pavoine, Purschke, Redding, Rosauer, Winter and Mazel2017). Both metrics were weighted by the species occupancy state in each sampling unit. The MPD is the observed average phylogenetic distance in a community and is defined by the length of the phylogeny branches among all possible taxa in the sample. The MNTD is the average phylogenetic distance to the nearest taxon within a community and is defined by the distance of the nearest taxon of each species within the phylogeny. To exclude species richness influence in our estimations, we estimated the standardised values that allow quantifying when MPD or MNTD are lower or higher than expected. We also employed null models and estimations from simulated assemblages for phylogenetic diversity metrics, as previously mentioned for functional diversity metrics. Negative values of standardised MPD and MNTD indicated assemblages where species are more related than expected: clustering. On the other hand, positive standardised MPD and MNTD suggest assemblages where species are less related than expected: overdispersion. Standardised values close to 0 indicate assemblages with random composition. The estimations were conducted using R (version 4.0, R Core Team, 2020) and the statistical package ape (Paradis and Schliep Reference Paradis and Schliep2019).
Statistical analysis
To evaluate the potential of A. ordinarium as a flagship species for amphibian conservation, we compared the values of functional and phylogenetic diversity between sampling units with and without detections of A. ordinarium. We employed a student’s t-test or Wilcoxon signed-rank test to compare values of the units with and without A. ordinarium. All analyses were carried out using R (version 4.0, R Core Team, 2020).
Results
From the 60 riparian sections in northeastern Michoacan, we used occupancy estimations of 20 amphibian species. We estimated that A. ordinarium occupied 21 of the 60 sampled stream sections. The species with the highest number of units occupied was the Pine Toad (Incillius occidentalis) with 53, while the Shiny Peeping Frog (Eleutherodactylus nitidus), the Dwarf Mexican Treefrog (Tlalocohyla smithii), and Rio Grande Leopard Frog (Lithobates berlandieri) occupied only one unit each. By sampling unit, we estimated that species richness oscillated from two to 10 amphibian species. Of the 21 sampling units occupied by A. ordinarium, species richness ranged from two to 10 species, while in those units without this salamander, species richness ranged from two to nine species.
Functional amphibian diversity
Since estimation of functional richness metrics requires data from at least three species, we used data from 56 of 60 sampling units. In riparian amphibian assemblages, FRich values ranged from 0.01 to 26.54. We estimated significantly higher values of FRich in sampling units where A. ordinarium was detected (mean ± SE = 10.7 ± 0.81) than where it was not (7.25 ± 0.86; W = 654, p < 0.001; Figure 2a). Standardised values of FRich, however, did not show differences between sampling units with A. ordinarium (-0.00 ± 0.11) and without it (-0.15 ± 0.14; W = 421, p = 0.30; Figure 2b). The FEve values ranged from 0.04 to 0.88, and there were significant differences in FEve between units where A. ordinarium was detected (0.68 ± 0.03) and where it was not (0.60 ± 0.03; W =476, p = 0.04; Figure 2c). In addition, standardised values of FEve were significantly higher (0.42 ± 0.25) in units with A. ordinarium detection than those without this salamander (-0.27 ± 0.17; t = 2.25, df = 36.45, p = 0.02; Figure 2d). The FDis values ranged from 0.42 to 4.00. In assemblages where A. ordinarium was detected, the FDis values were significantly higher (3.34 ± 0.11) as compared with assemblages where it was not detected (2.47 ± 0.08; W = 686, p < 0.001; Figure 2e). Similarly, standardised values of FDis were significantly higher in those units where A. ordinarium was detected (0.65 ± 0.10) in comparation with those where it is not (-0.52 ± 0.17; W = 699, p < 0.001; Figure 2f). The FDiv ranged from 0.56 to 0.96; however, there were no significant differences for FDiv between sampling units with A. ordinarium detections (0.76 ± 0.01) and without detections (0.77 ± 0.01; t = −0.53, df = 53.5, p = 0.59; Figure 2g). In addition, standardised values of FDiv did not differ between sampling units with A. ordinarium (-0.08 ± 0.16) and without it (0.09 ± 0.17; t = −0.73, df = 50.91, p = 0.46; Figure 2h).
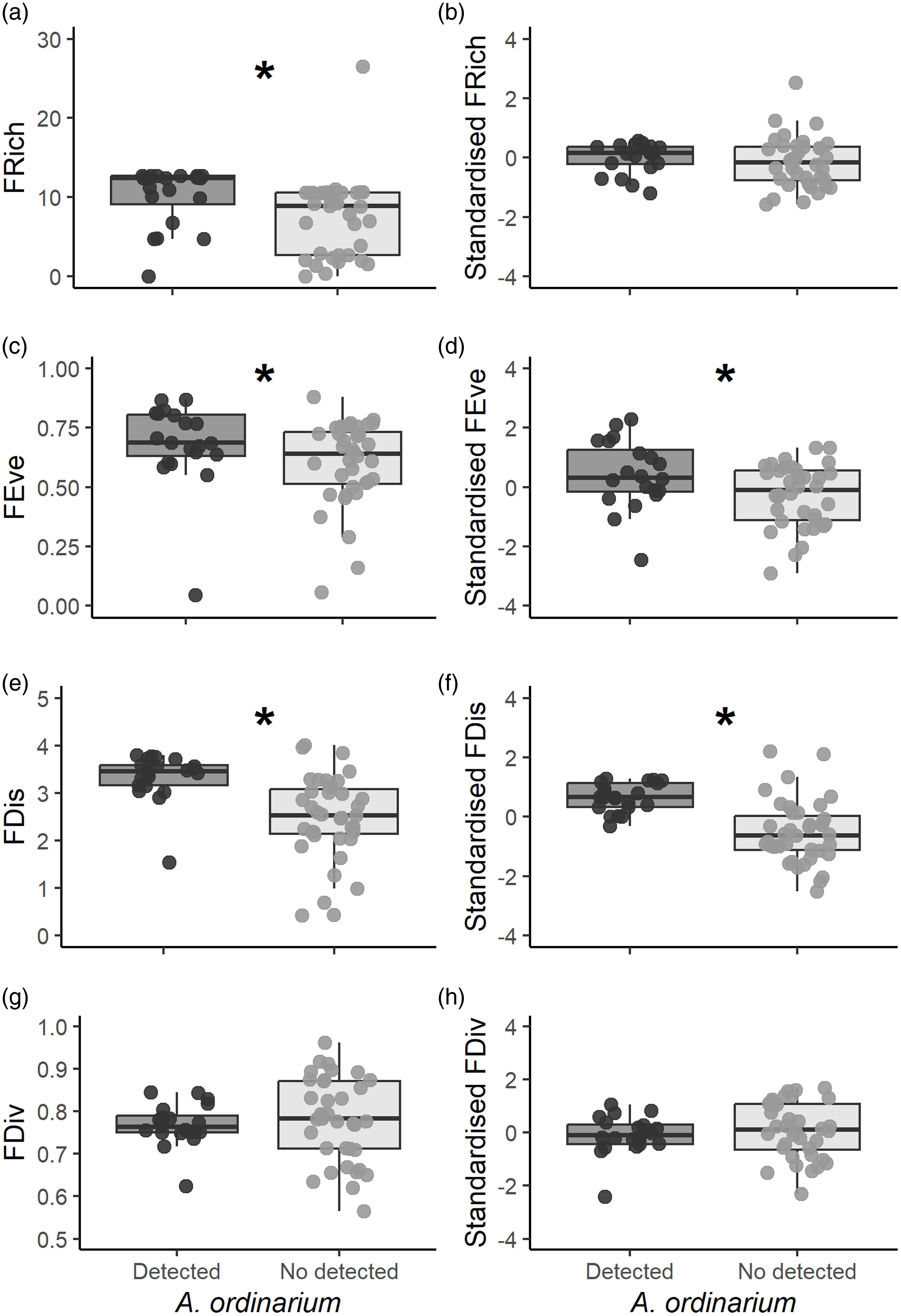
Figure 2. Estimated functional diversity for each sampling unit. We show the estimated values of functional richness, functional evenness, functional dispersion, functional divergence, and their standardised values. Graphs with an asterisk indicate significant differences among sampling units (p < 0.05).
Phylogenetic amphibian diversity
In the study zone, MPD values ranged from 24.25 to 318.28. In sampling units with A. ordinarium, we estimated significantly higher values of MPD (mean ± SE = 279.20 ± 8.59) than for those units without A. ordinarium (177.78 ± 10.15; t = 7.62, df = 56.68, p < 0.001; Figure 3a). For standardised MPD estimations, values ranged from −3.12 to 1.67, with significantly higher values for units with A. ordinarium (0.81 ± 0.10) as compared with units where this species was undetected (-0.62 ± 0.16; t = 7.47, df = 57.43, p < 0.001; Figure 3b). Values of MNTD ranged from 38.12 to 497.29, and we estimated higher values in units with A. ordinarium (206.7 ± 17.66) than in units where it was undetected (144.73 ± 10.87; t = 2.98, df = 35.34, p = 0.005; Figure 3c). Finally, the standardised MNTD ranged from −2.34 to 2.64. In units where A. ordinarium was detected, we found significantly higher values (0.84 ± 0.25) in comparation with units without detections (-0.81 ± 0.17; t = 5.35, df = 39.54, p < 0.001; Figure 3d).
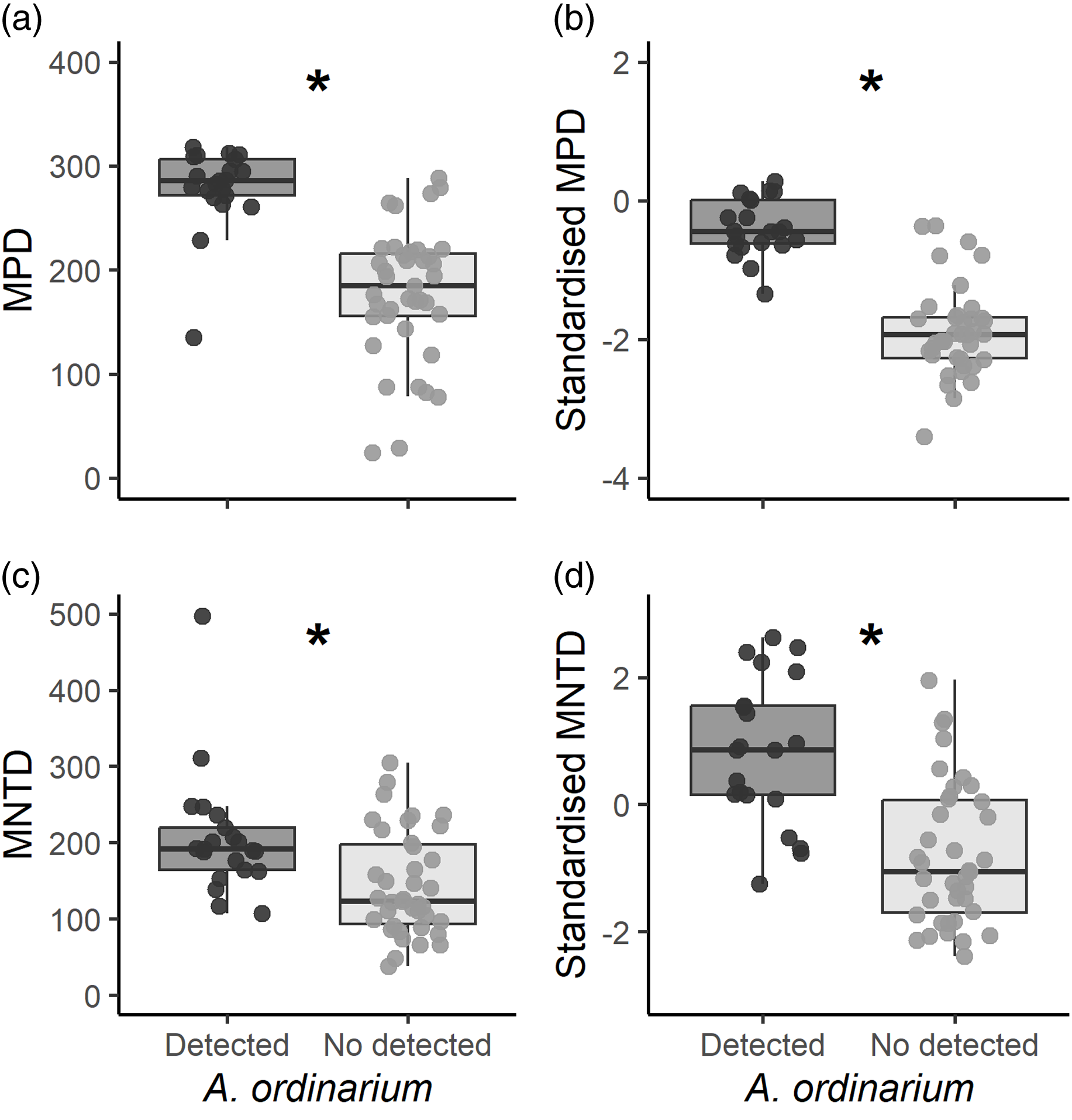
Figure 3. Estimated phylogenetic diversity for each sampling unit. We show estimated values of mean pairwise distance, mean distance to the nearest taxon, and their standardised values. Graphs with an asterisk indicate significant differences among sampling units (p < 0.05).
Discussion
Our results showed that functional diversity metrics and the phylogenetic diversity of amphibians in riparian habitats were higher in assemblages where A. ordinarium has been detected. These results support the proposal of using A. ordinarium as a flagship species to protect different facets of amphibian diversity and riparian zones that coincide with the distribution range of this threatened salamander.
Functional diversity in riparian zones inhabited by Ambystoma ordinarium
Because of species-specific habitat requirements (e.g., habitat specificity or optimal altitude), developing conservation strategies that consider all species assemblages with a wide variety of traits and differences in functional diversity is a major challenge (Dalerum Reference Dalerum2013, Srivathsa et al. Reference Srivathsa, Majgaonkar, Sharma, Singh, Punjabi, Chawla and Banerjee2020, Kim et al. Reference Kim, Park, Kim and Lee2021). This is evident in spatially heterogeneous regions where networks of protected areas are established under the criterion of unique species distribution. This approach may not be successful because of the geographic limitations of most emblematic species (Shen et al. Reference Shen, Li, McShea, Wang, Yu, Shi, Dong, Mi and Ma2019, Srivathsa et al. Reference Srivathsa, Majgaonkar, Sharma, Singh, Punjabi, Chawla and Banerjee2020). For example, to evaluate the effectiveness of four Chinese reserves that held species that differed in altitude preferences, conservation actions focused on the distribution area of four terrestrial vertebrates: the black muntjac (Muntiacus crinifrons), Elliot’s pheasant (Syrmaticus ellioti), giant panda (Ailuropoda melanoleuca), and takin (Budorcas taxicolor; Shen et al. Reference Shen, Li, McShea, Wang, Yu, Shi, Dong, Mi and Ma2019). Priority species narrowly co-occurred with the habitat of the four potential flagship species, however, making the flagship species’ proposals unfeasible. An alternative approach consists of defining the zones where threatened or high conservation priority species inhabit and then proposing the flagship species (e.g., Srivathsa et al. Reference Srivathsa, Majgaonkar, Sharma, Singh, Punjabi, Chawla and Banerjee2020, Kim et al. Reference Kim, Park, Kim and Lee2021). Because we have estimated that A. ordinarium occupied riparian with a high number of species at risk, we consider that our proposal for this salamander to be regarded as a flagship species has meaningful support for its application in northeastern Michoacán (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022).
Compared with modified environments, conserved habitats generally maintain higher species richness and species functions because of greater resource availability (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Santos, Pyron, Arroyo-Rodríguez, Urbina-Cardona, Martínez-Ramos, Parra-Olea and Reynoso2019, Strecker et al. Reference Strecker, Olden, Whittier and Paukert2011). Species assemblages, however, that include species with unique functional traits present higher functional richness than assemblages composed of species with shared functional traits (Jain et al. Reference Jain, Flynn, Prager, Hart, Devan, Ahrestani, Palmer, Bunker, Knops, Jouseau and Naeem2014, Leitão et al. Reference Leitão, Zuanon, Villéger, Williams, Baraloto, Fortunel, Mendonça and Mouillot2016). In northeastern Michoacán, we estimated higher FRich in riparian zones where A. ordinarium was detected, albeit this salamander inhabits modified zones and is restricted to elevations above 1900 m.a.s.l. This pattern could be due to the fact that high-altitude species assemblages included unique functional traits that were not shared with species in lower altitudes. For instance, the lungless salamanders Pseudoeurycea leprosa and Isthmura bellii (or A. ordinarium) recorded at and above 1900 m.a.s.l, do not share functional traits such as cutaneous breathing and their large size (i.e., Isthmura bellii) with lower-altitude amphibian species (Alvarado-Díaz et al. Reference Alvarado-Díaz, Suazo-Ortuño, Wilson and Medina-Aguilar2013). Furthermore, these two Plethodontidae salamanders are catalogued as ‘Threatened’ by the Mexican Government (SEMARNAT (Secretaría de Medio Ambiente y Recursos Naturales, México) 2019). Thus, the distribution area of A. ordinarium should be a priority for salamander conservation, for high-altitude amphibian species, and their unique functional traits. Because we estimated that FRich values were affected by species richness, exploring other functional diversity metrics can help to support and identify prioritised areas for conservation. Additionally, it cannot be assumed that zones with major species richness also present high functional diversity.
Contrary to modified environments, mature forests are commonly associated with higher values of functional evenness because of an assemblage structure less dominated by particular species traits (Maeshiro et al. Reference Maeshiro, Kusumoto, Fujii, Shiono and Kubota2013, Leitão et al. Reference Lemos-Espinal, Smith and Woolrich-Piña2018). When native species of mature forest and disturbance-tolerant species share traits (i.e., high functional redundancy), differences in FEve between environments are not observed (Luck et al. Reference Luck, Carter and Smallbone2013). In amphibians, FEve assessments between types of vegetation, land uses, or forest ages have not shown changes; this suggests a higher redundancy in amphibian functions (Díaz-García et al. Reference Díaz-García, Pineda, López-Barrera and Moreno2017, Alvarez-Grzybowska et al. Reference Alvarez-Grzybowska, Urbina-Cardona, Córdova-Tapia and García2020, Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Santos, Pyron, Arroyo-Rodríguez, Urbina-Cardona, Martínez-Ramos, Parra-Olea and Reynoso2019). It has been noted, however, that regional conditions such as precipitation (annual or seasonal), temperature and aridity can define the traits of the pool of amphibian species and thus the FEve of assemblages (Ochoa-Ochoa et al. Reference Ochoa-Ochoa, Mejía-Domínguez, Velasco, Marske and Rahbek2019). Thus, the higher values of FEve in riparian inhabited by A. ordinarium could be the result of suitable humidity and temperature conditions of more diverse amphibian traits in the Trans-Volcanic Mexican Belt, where this salamander of high-altitude riparian habitats is mostly distributed.
In the Sierra Madre del Sur, the tropical dry forest presents conditions that allow the establishment of dry-tolerant species (i.e., higher environmental temperature variation and lack of refuges with optimal humidity; Rzedowski Reference Rzedowski2006) and rarely will those species sensitive to humidity changes become highly abundant. Even in this type of vegetation, it is recognised that amphibian activity is almost restricted to the rainy season (Suazo-Ortuño et al Reference Suazo-Ortuño, Alvarado-Díaz and Martínez-Ramos2011, Alvarez-Grzybowska et al. Reference Alvarez-Grzybowska, Urbina-Cardona, Córdova-Tapia and García2020). In contrast, forests from the Trans-Volcanic Mexican Belt with more stable conditions could permit the entrance of species with different traits and not only those tolerant to dry conditions. Therefore, riparian habitats inhabited by A. ordinarium are markedly important as they sustain different amphibian functions and assemblages with a more stable structure.
Because A. ordinarium inhabits mature riparian forests as well as riparian zones bordering highly modified areas, the species is included in amphibian assemblages composed of highly disturbance-sensitive and disturbance-tolerant species regarding land-use change. In general, species tolerant to environmental disturbances share some traits (e.g., physiological or ethological) that allow population survival, the establishment of new populations, or a clustering pattern where species assemblages are integrated with functionally similar species (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Santos, Pyron, Arroyo-Rodríguez, Urbina-Cardona, Martínez-Ramos, Parra-Olea and Reynoso2019, Mori et al. Reference Mori, Furukawa and Sasaki2013, Menezes et al. Reference Menezes, Martins, Carvalho, Souza, Silveira, Loiola and Araújo2020).
The estimated functional dispersion for localities with A. ordinarium detection suggests that assemblages are composed of species with distinct functional traits. Therefore, the presence of disturbance-tolerant species could be the result of different adaptative traits that have allowed them to survive in modified habitats (Díaz-García et al. Reference Díaz-García, Pineda, López-Barrera and Moreno2017, Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Santos, Pyron, Arroyo-Rodríguez, Urbina-Cardona, Martínez-Ramos, Parra-Olea and Reynoso2019, Sol et al. Reference Sol, Trisos, Múrria, Jeliazkov, González-Lagos, Pigot, Ricotta, Swan, Tobias and Pavoine2020). Characteristics, such as higher temperature tolerance, a reproductive mode independent from vegetation structure, and dispersal events through streams, might enable species to cope with habitat modification. Such is the case of the tree frogs Dryophytes eximius and D. arenicolor, the leopard frog Lithobates neovolcanicus and A. ordinarium, which are dominant species in landscapes with a high proportion of croplands (Lemos-Espinal et al. Reference Lemos-Espinal, Smith and Woolrich-Piña2018, Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido and Munguía-Steyer2021). Consequently, the use of A. ordinarium as a flagship species could be useful for the conservation of amphibian assemblages with high functional distinctiveness, even in modified landscapes.
Phylogenetic diversity in A. ordinarium habitat
Less modified landscapes represent sources of species that help to maintain higher values of phylogenetic diversity even when the original vegetation is fragmented (Hernández-Ordóñez et al. Reference Hernández-Ordóñez, Santos, Pyron, Arroyo-Rodríguez, Urbina-Cardona, Martínez-Ramos, Parra-Olea and Reynoso2019, Ribeiro et al. Reference Ribeiro, Colli, Batista and Soares2017). Our estimations, however, showed high phylogenetic diversity in the A. ordinarium distribution area, even though it includes modified riparian zones. By comparing regions, a greater phylogenetic diversity of amphibians has been associated with higher precipitation and stable temperatures (Amador et al. Reference Amador, Soto-Gamboa and Guayasamin2019, Paúl et al. Reference Paúl, Rosauer, Tarroso, Velo-Antón and Carvalho2023). Therefore, higher values of phylogenetic diversity can be the result of greater precipitation and less temperature variation for the A. ordinarium habitat than in riparian zones where this salamander is absent.
Northeastern Michoacán is a biogeographically complex region, where the amphibian lineages of Nearctic (leopard frogs, salamanders and Dryophytes spp. tree frogs) and Neotropical origin (leaf litter frogs and Incilius spp. toads) are represented (Pauly et al. Reference Pauly, Hillis and Cannatella2004, Crawford and Smith Reference Crawford and Smith2005, Urbina-Cardona and Flores-Villela Reference Urbina-Cardona and Flores-Villela2010, Duellman et al. Reference Duellman, Marion and Hedges2016, Yuan et al. Reference Yuan, Zhou, Chen, Poyarkov, Chen, Jang-Liaw, Chou, Matzke, Iizuka, Min, Kuzmin and Che2016). Thus, the higher values of phylogenetic diversity could be the result of the A. ordinarium habitat including transition zones inhabiting both species with Nearctic and Neotropical origins. The Trans-Mexican Volcanic Belt assemblages can maintain a suitable environment for movement or species establishment from both regions (Alvarado-Díaz et al. Reference Alvarado-Díaz, Suazo-Ortuño, Wilson and Medina-Aguilar2013, Vega-Agavo et al. Reference Vega-Agavo, Suazo-Ortuño, Lopez-Toledo, Gómez-Tagle, Sillero, Pineda-López and Alvarado-Díaz2021). While in the Sierra Madre del Sur, a marked dry season could represent an unsuitable area for Nearctic amphibians, which are adapted to high elevation conditions (i.e., salamanders and Dryophytes spp. tree frogs). Thus, protecting the A. ordinarium habitat can be a suitable conservation strategy for protecting amphibians from different origins.
Phylogenetic and functional diversity can be useful tools in defining priority areas for conservation when taxonomic diversity (i.e., species richness) alone does not lead to conclusive information (Cadotte et al. Reference Cadotte, Albert and Walker2013, Forest et al. Reference Forest, Grenyer, Rouget, Davies, Cowling, Faith, Balmford, Manning, Procheş, Van Der Bank, Reeves, Hedderson and Savolainen2007, Strecker et al. Reference Strecker, Olden, Whittier and Paukert2011). For instance, in the Lower Colorado River Basin, in the United States, taxonomic, functional, and phylogenetic estimations were employed to identify priority areas for conserving native fishes (Strecker et al. Reference Strecker, Olden, Whittier and Paukert2011). By using only functional and phylogenetic metrics, it was possible to identify important conservation areas for native fishes, which did not represent a high priority when solely considering species richness. By using functional and phylogenetic metrics, it was possible to identify important areas for amphibian ecological functions and evolutionary history. Furthermore, contrary to species richness values, functional and phylogenetic diversity information supports the proposal of A. ordinarium as a flagship in northeastern Michoacán (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022).
The role of Ambystoma ordinarium as a flagship species
Considering that the sampling units in which A. ordinarium was recorded showed a higher number of species at risk, we previously suggested that this salamander could be used as a flagship species (Oropeza-Sánchez et al. Reference Oropeza-Sánchez, Suazo-Ortuño, Benítez-Malvido, Monroy-Hernández and Munguía-Steyer2022). With the present results, the high values of functional and phylogenetic diversity reinforce the proposal of A. ordinarium as a flagship species. There are lower-altitude amphibian species at high extinction risk; therefore, implementing complementary conservation strategies could prevent future threats to this species group and its habitats.
For the conservation of biological diversity, various strategies in addition to flagship species have been used. For example, prioritise the protection of species with a high extinction risk. In the Republic of South Korea, species distribution modelling was used to conserve the white-naped crane (Antigone vipio) habitat, also providing protection to other species of waterbirds and even species with different ecological traits (Kim et al. Reference Kim, Park, Kim and Lee2021). Moreover, employing multiple flagship species seems a reasonable alternative in complex geographic regions (Shen et al. Reference Shen, Li, McShea, Wang, Yu, Shi, Dong, Mi and Ma2019). For instance, in India, it was estimated that protecting the distribution area of nine flagship carnivore species (eight canids and hyaenas) would lead to higher benefits for background species and vulnerable habitats than the current protected area network (Srivathsa et al. Reference Srivathsa, Majgaonkar, Sharma, Singh, Punjabi, Chawla and Banerjee2020). Multiple flagship species could be a reliable way to preserve northeastern Michoacán amphibians, while protected natural areas or more elaborate conservation strategies are adopted. To give provisional protection to amphibians outside the A. ordinarium distribution area, it would be important to protect species at lower altitudes or those within the Sierra Madre del Sur, after assessing the coincidence of high diversity values.
In addition to its biological relevance, the rationale for the use of A. ordinarium as a flagship species is strengthened by the cultural importance of the Ambystoma species (axolotls) in Mexico. Several Ambystoma species have been considered as deities and used for food since prehispanic times (Soto-Rojas and Suazo-Ortuño Reference Soto-Rojas and Suazo-Ortuño2019). Additionally, axolotls have been used to treat health issues associated mainly with respiratory illness and melancholic states of mind (Velarde-Mendoza Reference Velarde-Mendoza2012; Díaz-García et al. Reference Díaz-García, Pineda, López-Barrera and Moreno2017). Therefore, of all the riparian amphibian species, A. ordinarium is the most recognised and most regarded as beneficial by the human communities living within the distribution range of A. ordinarium. The main conservation threats of A. ordinarium are associated with the destruction and degradation of mountain streams and adjacent riparian habitats. Therefore, incorporation of local communities in the planning and implementation of actions for the conservation of riparian habitats and species is feasible if A. ordinarium is used as a symbol for this conservation effort.
Because of the limited dispersal of amphibians, conservation strategies must take into consideration the establishment of vegetation corridors. It will be important for these corridors to connect riparian habitats in modified landscapes to those with native forest. This can allow the availability of favourable environmental conditions for the preservation of species richness and the functional diversity of amphibians (Todd and Winne Reference Todd and Winne2006, Santos-Barrera and Urbina-Cardona Reference Santos-Barrera and Urbina-Cardona2011).
Conclusions
Our results showed that sampling units with the presence of A. ordinarium presented high phylogenetic and functional diversity of amphibian assemblages. Therefore, this salamander can be considered a flagship species as part of a conservation strategy for amphibians in the study region. To make this possible, we will need complementary information regarding amphibian functions and the status of their populations in modified landscapes. Consequently, we must assess other population traits, such as biotic interactions, survival rates, reproductive success, and the colonisation capability of different amphibian species.
Acknowledgements
We thank Erandi Monroy-Hernández, Julio Rosales-Vilchis, Andony Olmos-Mercado, Yunuen Soto-Sandoval, Mario Alberto Sosa-Toche, Patricia Hernández-Lopez, Miguel Piñon, Cinthya Mendoza-Almeralla, Alfredo Camarillo, and Andrea Raya for their help in the field search for amphibians; we also thank the local people for their permission and support. We also thank Juan Manuel Lobato-García and Jonatan Torres Pérez Coeto for their technical and logistical support. Additionally, we thank Ellen Andresen for her comments on this manuscript and Juan Manuel Díaz-García for his comments on the analysis section. Marco Tulio Oropeza-Sánchez obtained a scholarship from CONACyT (623120), Mexico. We are also grateful for the support (infrastructure, logistics, and administration team) provided by the Instituto de Investigaciones en Ecosistemas y Sustentabilidad (IIES-UNAM) and the Universidad Michoacana de San Nicolás de Hidalgo. The results of this study are part of the PhD thesis of the corresponding author, under the direction of ISO.
Financial support
This research was funded by the Comisión Nacional de Ciencia y Tecnología (CONACyT, number 259173) and a Rufford Small Grant (27008-1). This study was part of the project ‘Efecto de la calidad del agua sobre parámetros poblacionales, fisiológicos y morfológicos de la salamandra de montaña (Ambystoma ordinarium)’, from the Secretaría de Educación Pública/Consejo Nacional de Ciencia y Tecnología Ciencia Básica 2015- 259173.
Competing interests
The authors declare none.
Ethical statement
This study complies with ethical standards. The scientific collecting permit number SGPA/DGVS/001450/18 was granted by SEMARNAT. We employed the guidelines for use of live amphibians in the field and laboratory research subscribed by the American Society of Ichthyologists and Herpetologists were followed for the collection and handling of specimens (2004).







