Article contents
Microanalysis of the Intestinal Bulb of Grass Carp (Ctenopharyngodon Idella): Histological, Histochemical, Immunohistochemical, and Scanning Electron Microscopical Studies
Published online by Cambridge University Press: 06 October 2021
Abstract
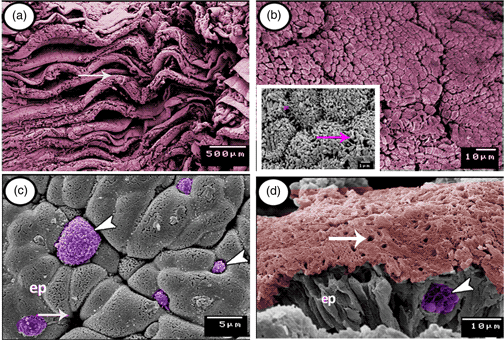
Cyprinid fishes have one of the simplest types of gastrointestinal tract among vertebrates. Those fish species do not possess a true stomach that is replaced by a simple dilatation at the anterior part of the intestine called the intestinal bulb. Twenty adult specimens of grass carp were used in the present study to identify the cellular components as well as the immunohistochemical and surface architectural characteristics of the intestinal bulb. The mucosa of the intestinal bulb shows numerous, deep longitudinal folds arranged in zigzagging-like patterns. The epithelium is composed mainly of absorptive columnar cells covered by microvilli and mucous goblet cells. Spindle-shaped enteroendocrine cells and some migratory immune cells such as intraepithelial lymphocytes and rodlet cells could be identified between the absorptive cells. The epithelium also contains many secretory granules and large numbers of vacuoles containing digestive enzymes mostly in the basal part. The immunohistochemistry revealed that CD20-positive B-lymphocytes are immunolocalized mainly in the connective tissue core lamina propria of the mucosal folds. However, CD3-immunopositive T-lymphocytes are highly concentrated in the lamina propria. In addition, intraepithelial T-lymphocytes expressed immunopositivity to CD3. The current study presented many types of immune cells and suggests their essential immunological role for the intestinal blub.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 2
- Cited by





