Article contents
Preemptive Stem Cells Ameliorate Neuropathic Pain in Rats: A Central Component of Preemptive Analgesia
Published online by Cambridge University Press: 16 February 2021
Abstract
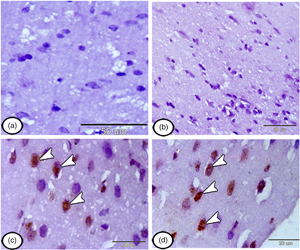
The present study aims to investigate the efficacy of intravenously injected mesenchymal stem cells (MSCs) in treating neuropathic pain either before or after its induction by a chronic constriction injury (CCI) model. Rats were divided into four groups: control group, neuropathic group, and treated groups (pre and postinduction) with i.v. mononuclear cells (106 cell/mL). For these rats, experimental testing for both thermal and mechanical hyperalgesia was evaluated. The cerebral cortex of the rats was dissected, and immunohistochemical analysis using anti-proliferating cell nuclear antigen (PCNA), CD117, nestin, and glial fibrillary acidic protein was performed. Our results showed that a single injection of MSCs (either preemptive/or post-CCI) produced equipotent effects on allodynia, mechanical hyperalgesia, and thermal response. Immunohistochemical analysis showed that the stem cells have reached the cerebral cortex. The injected group with MSCs before CCI showing few stem cells expressed PCNA, CD117, and nestin in the cerebral cortex. The group injected with MSCs after CCI, showing numerous recently proliferated CD117-, nestin-, PCNA-positive stem cells in the cerebral cortex. In conclusion, our findings demonstrate that the most probable effect of i.v. stem cells is the central anti-inflammatory effect, which opens concerns about how stem cells circulating in systemic administration to reach the site of injury.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 5
- Cited by





