Article contents
Spatial Image Resolution Assessment by Fourier Analysis (SIRAF)
Published online by Cambridge University Press: 03 March 2022
Abstract
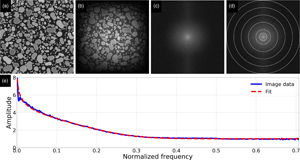
Determining spatial resolution from images is crucial when optimizing focus, determining smallest resolvable object, and assessing size measurement uncertainties. However, no standard algorithm exists to measure resolution from electron microscopy (EM) images, though several have been proposed, where most require user decisions. We present the Spatial Image Resolution Assessment by Fourier analysis (SIRAF) algorithm that uses fast Fourier transform analysis to estimate resolution directly from a single image without user inputs. The method is derived from the underlying assumption that objects display intensity transitions, resembling a step function blurred by a Gaussian point spread function. This hypothesis is tested and verified on simulated EM images with known resolution. To identify potential pitfalls, the algorithm is also tested on simulated images with a variety of settings, and on real SEM images acquired at different magnification and defocus settings. Finally, the versatility of the method is investigated by assessing resolution in images from several microscopy techniques. It is concluded that the algorithm can assess resolution from a large selection of image types, thereby providing a measure of this fundamental image parameter. It may also improve autofocus methods and guide the optimization of magnification settings when balancing spatial resolution and field of view.
- Type
- Software and Instrumentation
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 3
- Cited by





