Article contents
Auroselenide, AuSe, a new mineral from Maletoyvayam deposit, Kamchatka peninsula, Russia
Published online by Cambridge University Press: 19 December 2022
Abstract
Auroselenide, ideally AuSe, is a new mineral from the Gaching ore occurrence of the Maletoyvayam deposit, Kamchatka peninsula, Russia. It occurs as anhedral grains up to 0.05 × 0.02 mm and as intergrowths up to 0.06 mm with maletoyvayamite–tolstykhite-series minerals, enclosed in native gold. Other associated minerals include pyrite, calaverite, fischesserite, gachingite, tetrahedrite-group minerals [stibiogoldfieldite, its As-analogue, tennantite-(Cu) and tetrahedrite-(Zn)], tripuhyite, minerals of the famatinite–luzonite and selenium–tellurium series, paraguanajuatite, petrovskaite, součekite and tiemannite. Auroselenide is bluish-grey, opaque with metallic lustre and grey streak. It is brittle and has an uneven fracture. Dcalc = 9.750 g/cm3. In reflected light, auroselenide is grey with a bluish shade. Bireflectance is very weak. No pleochroism and internal reflections are observed. In crossed polars, it is strongly anisotropic with bluish to brownish rotation tints. The reflectance values for wavelengths recommended by the Commission on Ore Mineralogy of the International Mineralogical Association are (Rmin/Rmax, %): 28.4/31.5 (470 nm), 30.2/33.3 (546 nm), 31.9/34.9 (589 nm) and 34.3/37.3 (650 nm). The principal bands in the Raman spectrum of auroselenide are at 93, 171, 200, 210 and 325 cm–1. The empirical formula calculated on the basis of 2 atoms per formula unit is (Au0.98Ag0.01)Σ0.99(Se0.79S0.17Te0.05)Σ1.01. Auroselenide is monoclinic, space group C2/m, a = 8.319(1), b = 3.616(1), c = 6.276(2) Å, β = 104.54(2)°, V = 182.74(5) Å3 and Z = 4. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 4.015 (54) (200); 3.033 (25) (${\bar 1}$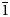 11, 002); 2.780 (100) (${\bar 2}$
11, 002); 2.780 (100) (${\bar 2}$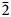 02, 111); 2.172 (20) (${\bar 3}$
02, 111); 2.172 (20) (${\bar 3}$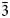 11, 310); and 1.811 (25) (${\bar 1}$
11, 310); and 1.811 (25) (${\bar 1}$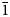 13). Auroselenide is the natural analogue of synthetic β-AuSe. The structural identity between them is confirmed by powder X-ray diffraction and Raman spectroscopy. The mineral is named according to its composition, as a combination of the main elements Au (aurum) and Se (selenium).
13). Auroselenide is the natural analogue of synthetic β-AuSe. The structural identity between them is confirmed by powder X-ray diffraction and Raman spectroscopy. The mineral is named according to its composition, as a combination of the main elements Au (aurum) and Se (selenium).
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: František Laufek
References
- 3
- Cited by




