No CrossRef data available.
Article contents
MgSiO3 perovskite: a HRTEM study
Published online by Cambridge University Press: 05 July 2018
Abstract
Selected-area electron diffraction patterns for the [110] zone of MgSiO3 perovskite are consistent with the orthorhombic unit cell obtained by X-ray diffraction (a = 4.775, b = 4.929, c = 6.897 Å). Various areas of a crystal fragment show diffuse streaking along c*, and well-developed satellite reflections that give a 3-fold repeat along [1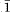 0]*. Another fragment shows doubled cell dimensions when viewed down [
0]*. Another fragment shows doubled cell dimensions when viewed down [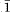 30]. The variable occurrence of the satellite reflectioncs and diffuse streaking indicate subtle variations in ordering, chemistry, or both. Images obtained by high-resolution transmission electron microscopy contain perfectly ordered regions, out-of-phase boundaries, and intergrowths of the two orthorhombic forms of perovskite.
30]. The variable occurrence of the satellite reflectioncs and diffuse streaking indicate subtle variations in ordering, chemistry, or both. Images obtained by high-resolution transmission electron microscopy contain perfectly ordered regions, out-of-phase boundaries, and intergrowths of the two orthorhombic forms of perovskite.
Keywords
- Type
- Mineralogy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1996




