Article contents
Electrode architecture of carbon-coated silicon nanowires through magnesiothermic reduction for lithium-ion batteries
Published online by Cambridge University Press: 09 October 2017
Abstract
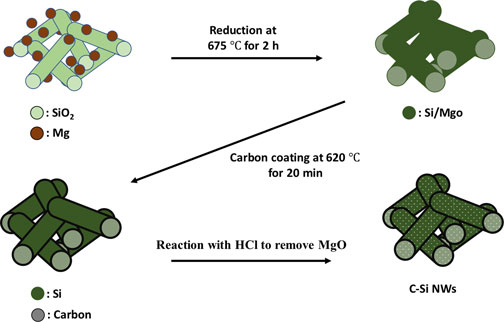
Carbon-coated silicon nanowires (C-Si NWs) were prepared as anodes for lithium-ion batteries (LIBs). The C-Si NWs were synthesized using a simple and effective fabrication strategy via magnesiothermic reduction. The synthesis sequence of carbon coating before the chemical etching of the reduced Si NWs/MgO composite was found to be critical for improved battery performance. In addition, carbon coating was found to help to stabilize the solid electrolyte interphase layer during battery cycling, which is important to realize the benefits of Si-based LIBs. This synthesis method provides an efficient route to synthesizing high-performance Si electrodes via magnesiothermic reduction.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 4
- Cited by





