Article contents
Examining the interlayer interactions formed between reduced graphene oxide and ionic liquids
Published online by Cambridge University Press: 01 March 2013
Abstract
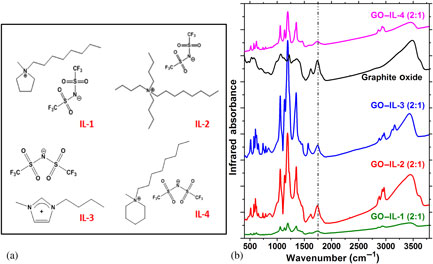
It is important to understand the electrolyte–electrode interactions for fabricating graphene oxide (GO)- and ionic liquid (IL)-based ultracapacitors. Therefore, we explored how the type and size of the cations in various ILs determine the nature of processed materials. In all cases, the ILs intercalate into the graphitic structure but marked differences are observed during exfoliation via thermal reduction. The combination of a long alkyl chain ammonium-based cation and a large-volume anion leads to strong interactions and defect formation, as evidenced by CO2 production during annealing. In contrast, using the same anions but different cations stabilize the GO functional groups below 400 °C.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
- 1
- Cited by




