Article contents
Modulated nitric oxide delivery in three-dimensional biomaterials for vascular functionality
Published online by Cambridge University Press: 19 June 2017
Abstract
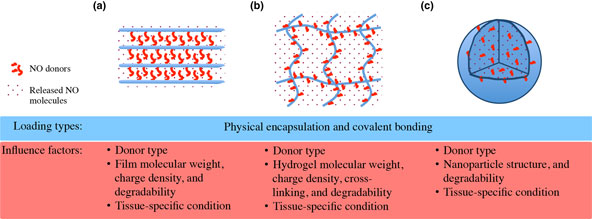
Nitric oxide (NO) acts a pivotal role in regulating various physiological processes of vasodilation, platelet aggregation, and vascular smooth muscle cell mitogenesis and proliferation. This makes NO a promising candidate for the treatment of cardiovascular problems like hypertension and vascular stenosis. However, the high reactivity of NO poses an issue for effective NO delivery. To overcome this limitation, recent developments on three-dimensional (3D) materials have been explored with either physical or chemical incorporation of NO releasing donors, to provide spatiotemporal control over NO-signaling pathways in blood vessels. Here, we offer an overview on the current efforts, and propose future perspectives for precise regulation on NO delivery in advanced 3D materials toward proper vascular functionality.
- Type
- Biomaterials for 3D Cell Biology Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
These authors contributed equally to this work.
References
- 4
- Cited by




