Article contents
Molecular phylogeny and new light microscopic data of Metchnikovella spiralis (Microsporidia: Metchnikovellidae), a hyperparasite of eugregarine Polyrhabdina sp. from the polychaete Pygospio elegans
Published online by Cambridge University Press: 12 April 2021
Abstract
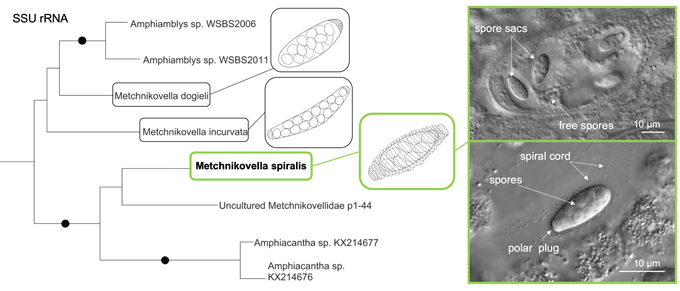
Metchnikovellids are a deep-branching group of microsporidia, parasites of gregarines inhabiting the alimentary tract of polychaetes and some other invertebrates. The diversity and phylogeny of these hyperparasites remain poorly studied. Modern descriptions and molecular data are still lacking for many species. The results of a light microscopy study and molecular data for Metchnikovella spiralis Sokolova et al., 2014, a hyperparasite of the eugregarine Polyrhabdina sp., isolated from the polychaete Pygospio elegans, were obtained. The original description of M. spiralis was based primarily on the analysis of stained preparations and transmission electron microscopy images. Here, the species description was complemented with the results of in vivo observations and phylogenetic analysis based on the SSU rRNA gene. It was shown that in this species, free sporogony precedes sac-bound sporogony, as it occurs in the life cycle of most other metchnikovellids. Spore sacs are entwined with spirally wound cords, and possess only one polar plug. Phylogenetic analyses did not group M. spiralis with M. incurvata, another metchnikovellid from the same gregarine species, but placed it as a sister branch to Amphiacantha. The paraphyletic nature of the genus Metchnikovella was discussed. The taxonomic summary for M. spiralis was emended.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 2
- Cited by





