The brain is largely composed of fat(Reference Pakiet, Jakubiak and Czumaj1), and it is now clear that membrane lipids play essential roles in the brain, as structural elements, but also as signalling molecules. Recent data pinpoint the crucial role of lipids in the functioning of the synapse, the basic structural and operational unit for information processing and storage in the brain. The lipid composition of the synapse is distinct from the rest of the cell membranes. Indeed, synaptic membrane lipids are crucial to neurotransmitter receptors stabilisation and activity, neurotransmitter vesicle release and synapses formation and development (recently reviewed in(Reference Postila and Róg2)). Among lipids with critical roles at the synapse, PUFA are the major actors for synaptogenesis, synapse maintenance and synapse function. These specific fatty acids, especially the long chain (LC) PUFA (Fig. 1), are particularly concentrated in the brain(Reference Sastry3). Arachidonic acid (ARA; 20:4, n-6) and DHA (22:6, n-3), which belong to distinct PUFA species (n-6 and n-3 PUFA, respectively), are the major LC PUFA found in the brain(Reference Bazinet and Layé4–Reference McNamara, Jandacek and Rider6). ARA and DHA reach the brain through passive diffusion or active transporters. They are then esterified into distinct phospholipids (recently reviewed in(Reference Lacombe, Chouinard-Watkins and Bazinet7)). Importantly, PUFA are essential fatty acids, meaning that they cannot be synthesised in the body, while needed for conservation of health, and therefore require dietary supply. Linoleic acid (LA; 18:2, n-6), the precursor of ARA, and α-linolenic acid (18:3, n-3), the precursor of EPA and DHA, are found in distinct plant and oils and, once ingested, are desaturated and elongated in the body to form the respective LC n-6 and n-3 PUFA. Preformed ARA, EPA and DHA are also provided through the diet from distinct terrestrial and marine animal sources. Overall, the consumption of n-3 PUFA is lower than the actual dietary recommendation for α-linolenic acid, EPA and DHA(8) with an omega-3 index (% of EPA + DHA in erythrocytes, which reflects the dietary intake) below the defined adequate amount (>8 %), which constitutes a risk factor for CVD and brain diseases(Reference Kones, Howell and Rumana9). Dietary recommendations for LC n-3 PUFA as well as total n-3 PUFA have been reviewed in a previous study(Reference Molendi-Coste, Legry and Leclercq10). Of note, LA is one of the most consumed n-6 PUFA, as recently revealed by westernised countries diet composition(Reference Blasbalg, Hibbeln and Ramsden11).
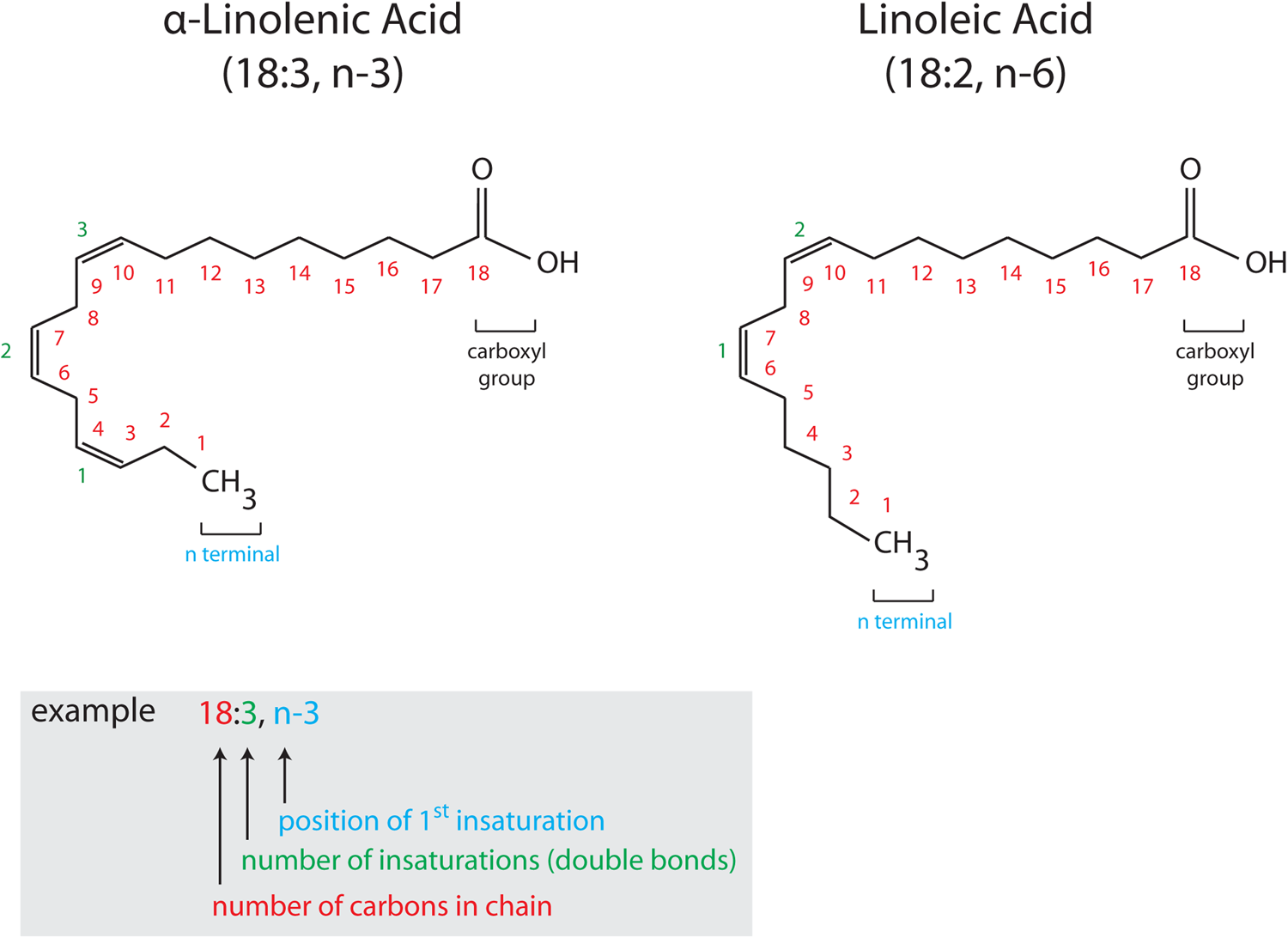
Fig. 1. (Colour online) Structure and nomenclature of PUFA. PUFA are composed of a hydrocarbon chain (CH2) with a methyl group (CH3, α terminal) at one extremity and a carboxyl group (COOH, ω terminal) at the other. Unsaturations are found along the hydrocarbon chain (represented by double bonds). Chemical formulas of PUFA are commonly presented in a simplified way, X:Y, n-6 or n-3, where X indicates the number of carbon atoms and Y corresponds to the number of double bonds. n-6 and n-3 indicate the number of the carbon atom of the first double bond (6 or 3), with n indicating that the double bonds are counted from the CH3 terminal. α-Linolenic acid (18:3, n-3) and linoleic acid (18:2, n-6) are drawn as two representative examples of n-3 and n-6 PUFA, respectively with the nomenclature detailed in the colour-coded inset.
Accumulating data in human subjects linked brain pathologies presenting synaptic or deficits with dietary habits in PUFA(Reference Samieri, Féart and Letenneur12). The role of ARA and DHA at the synapse in physiological and pathological contexts has recently generated a growing interest. Indeed, disruption in PUFA metabolism emerges as an important factor for some neurodevelopmental and neuropsychiatric disorders and neurodegenerative diseases(Reference Bazinet and Layé4,Reference Bazinet, Metherel and Chen13–Reference Messamore and McNamara15) . Following these observations, clinical trials have been conducted to improve or delay some of these pathologies using LC n-3 PUFA, which produced mixed results, depending on the type and dose of LC n-3 PUFA, length of treatment, as well as the targeted pathologies(Reference Bazinet, Metherel and Chen13,Reference Delrieu, Payoux and Carrié16–Reference Chianese, Coccurello and Viggiano18) . This pinpoints the need for a better understanding of the basic mechanisms linking PUFA and synapse function, in order to identify efficient therapeutic approaches.
This review aims at highlighting direct and indirect roles of ARA and DHA at the synapse. We describe how ARA and DHA are involved in synaptogenesis and how they control synaptic transmission as well as synaptic plasticity. The possible underlying mechanisms are presented, even though research in this recent field is still largely ongoing.
The concept of synaptopathies
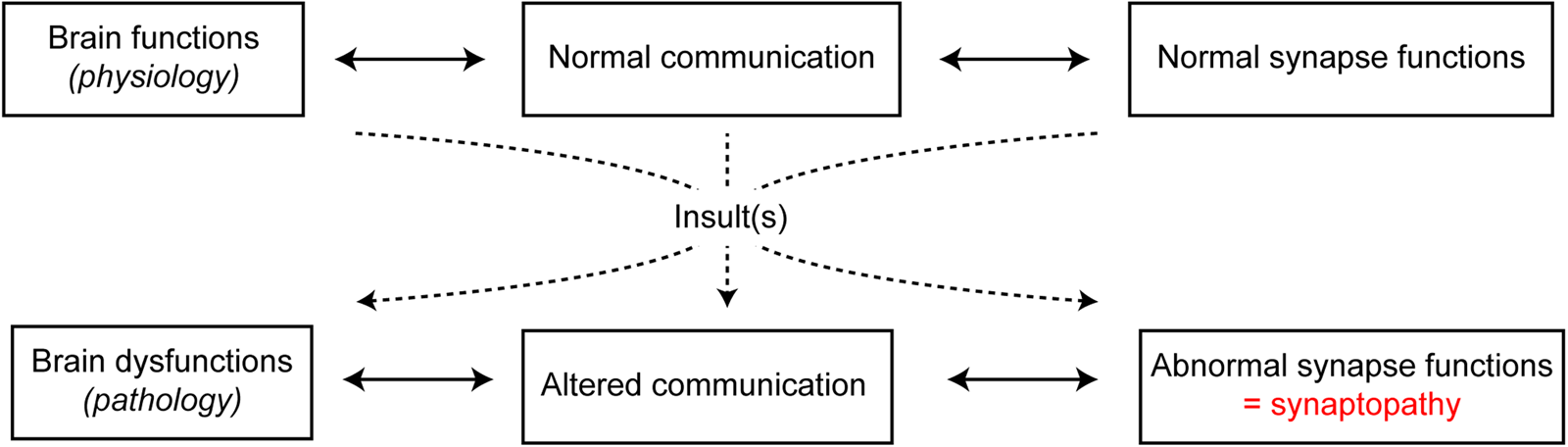
Adequate development and maintenance of synapses are essential for normal functioning of the nervous system. In the past few years, the concept that brain disorders should not be dichotomised into early-onset neurodevelopmental and late-onset neurodegenerative disorders has emerged. Indeed, it should be rather considered that pathologies share common features, such as perturbation of neuronal activity, particularly at the synaptic level. In fact, several brain diseases with pathophysiological processes, even if distinct, ultimately lead to synaptic impairments that are referred to as synaptopathies(Reference Pozzi, Menna and Canzi19) (Fig. 2). This is the case for neurodevelopmental disorders such as autism spectrum disorders and schizophrenia, for neuropsychiatric disorders such as depression, and for neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, multiple sclerosis and Huntington's disease. Indeed, all of these neurological disorders are characterised by synaptic dysfunctions(Reference Imbriani, Schirinzi and Meringolo20–Reference Betancur, Sakurai and Buxbaum23). These observations lead to the idea that synaptic damage should be considered as a target for neurological and neuropsychiatric disease-modifying treatments(Reference Pozzi, Menna and Canzi19,Reference Monday and Castillo24,Reference Crisp, Kullmann and Vincent25) . Synapses are highly dynamic and plastic. This is illustrated by the dynamics of synaptic networks over time during learning processes with electrophysiological synaptic plasticity, old synapses replaced by new ones and differences in the number of spines in stimulated neuronal networks(Reference Holtmaat and Svoboda26). Interestingly, these synaptic properties support the idea that aberrant synapses can be replaced by new and functional synapses, as seen during synaptic plasticity or rehabilitative processes(Reference Morgen, Kadom and Sawaki27). Therefore, the understanding of overlapping mechanisms in synapse dysfunctions is of high interest. In this frame, investigating the role of PUFA in synapse function and dysfunction is necessary in order to design new pharmaceutical tools to protect, improve or optimise synaptic functions.

Fig. 2. (Colour online) Transition from physiological to pathological brain functions by synaptopathies. Under healthy physiological conditions, neuronal communication and synaptic functions are normally functional. However, under pathological conditions, following one or more insults, altered communication is paralleled with abnormal synapse functions, referred to as synaptopathies.
The role of PUFA in synapse formation
In the brain, ARA and DHA accumulate predominantly during development, into brain cells including neurons and glial cells such as astrocytes and microglia(Reference Rey, Nadjar and Joffre28). ARA and DHA provided by the mother to the fetus and newborn are directly associated with maternal dietary intake and body stores. As a result, pregnant and lactating women store LC PUFA to ensure an adequate flow of ARA and DHA to the fetus and newborn(Reference Lauritzen and Carlson29,Reference Larqué, Gil-Sánchez and Prieto-Sánchez30) . Importantly, PUFA are transported from the mother to the newborn through the placenta and the milk, respectively. Human breast milk contains both DHA and ARA, the levels of which depend on the mother's diet(Reference Bravi, Wiens and Decarli31). The presence of endocannabinoids (eCB) and oxylipins has also been found in maternal milk, suggesting that not only PUFA are transferred to the newborns, but also their derivatives(Reference Wu, Domellöf and Zivkovic32). This early-life accumulation raises the importance of adequate ARA and DHA dietary supplies during perinatal periods, as previously reviewed(Reference Innis33). In addition, most children do not meet dietary guidelines for LC n-3 PUFA, reflected by a poor omega-3 index, a risk factor for altered brain maturation and possibly synaptopathies of neurodevelopmental origin(Reference McNamara, Jandacek and Tso34). However, increased oily fish consumption in children and dietary supplementation of pregnant women with LC n-3 PUFA are encouraging strategies(Reference Vuholm, Rantanen and Teisen35,Reference Gould, Treyvaud and Yelland36) .
During brain development, ARA and DHA influence neurogenesis, synaptogenesis, neuronal migration and neuronal differentiation(Reference Lauritzen, Brambilla and Mazzocchi37). In rodents, perinatal dietary n-3 PUFA deficiency decreases brain DHA and concomitantly reduces neurogenesis, synaptic plasticity and connectivity(Reference Cao, Kevala and Kim38–Reference Yavin, Himovichi and Eilam42). Perinatal dietary n-3 PUFA deficiency impairs synaptic pruning, the selective phagocytosis of spines by microglia during brain development(Reference Paolicelli, Bolasco and Pagani43), in the visual system and the hippocampus(Reference Madore, Nadjar and Delpech44,Reference Madore, Leyrolle and Morel45) . The mechanisms underlying the crucial role of DHA in brain development and neuronal network formation is still poorly understood. In vitro, DHA promotes neurite outgrowth, synaptic functions and maintenance(Reference Calderon and Kim46). Diets rich in DHA promote dendritic spine density and protect from dendritic deficits(Reference Calon, Lim and Yang47). Moreover, DHA strongly influences the dynamics of membrane phosphatidylserine, which promotes membrane formation and is a necessary step for additional synapses formation(Reference Hamilton, Greiner and Salem48). Recent data identified that N-docosahexaenoylethanolamide (DHEA), a DHA-derived eCB, could be the bioactive pro-synaptogenesis molecular intermediate through its activity on an orphan receptor, GPR110(Reference Kim, Moon and Cao49,Reference Lee, Huang and Kwon50) . DHEA activity on GPR110 has also been reported to counteract neuro-inflammation(Reference Park, Chen and Kim51). Interestingly, DHEA seems to be more active in the enhancement of synaptogenesis than DHA.
The observation that altered n-3 PUFA bioavailability and activity in the developing brain participate to an altered brain development and impaired neuronal network and synaptic function suggests an aetiological role of these fatty acids in neurodevelopmental synaptopathies. Low levels of DHA and/or EPA have been reported in children with autism spectrum disorders, attention deficit and hyperactivity disorder or schizophrenia(Reference Messamore and McNamara15,Reference Chang, Su and Mondelli52) . Recently, several clinical trials have been performed to supplement patients with neurodevelopmental disorders with DHA and/or EPA, with some beneficial effects when administered alone or in combination with other nutrients/micronutrients(Reference Adams, Audhya and Geis53). The positive effect of an EPA monotherapy was greater in attention deficit and hyperactivity disorder patients with low endogenous EPA at the start of the supplementation(Reference Chang, Su and Mondelli54), similarly to what has been described in patients diagnosed with major depression(Reference Mischoulon, Papakostas and Dording55). This reinforces the importance of investigating PUFA levels in children diagnosed with neurodevelopmental disorders to develop more personalised PUFA supplementation strategies.
Modulation of neurotransmission by n-3 and n-6 PUFA
Neurotransmission is the process by which neurons transmit electric signals (Fig. 3A). Brain cellular membranes are a reservoir of LC PUFA from the n-6 and n-3 families, which are released by specific phospholipases ((PL)A2) (Fig. 3B). Importantly, these PLA2 are stimulated by neurotransmission and several neurotransmitters, leading to a release of free LC PUFA that are bioactive messengers, which, in turn, could regulate neurotransmission(Reference Bazan56). Indeed, many studies focused on the effects of ARA, DHA or EPA and some of their derivatives on neurotransmission. To study the effect of bioactive PUFA/derivatives on neurotransmission, several approaches have been used: (i) application of free LC PUFA on neurons (in vitro or on ex vivo brain slices) belonging to several neurotransmitter systems such as excitatory glutamate, inhibitory γ aminobutyric acid (GABA), or neuromodulatory dopamine; (ii) dietary manipulation of amount of PUFA in brain cell membrane to change the amount of neuronal membrane PUFA and/or bioavailability of endogenous free PUFA during neurotransmission. These studies are summarised later. ARA, EPA and DHA are also known to regulate some brain processes through oxylipins, the oxidised metabolites produced or synthesised via lipoxygenase, cyclooxygenase (COX), cytochrome P450 or soluble epoxide hydrolase enzymes following their PLA2-mediated release from brain membrane phospholipids(Reference Layé, Nadjar and Joffre57–Reference Bosch-Bouju and Layé60) (Fig. 3B). Of note, recent data pinpointed that oxidised LA derivatives, triggered by ischaemia, are potent modulators of neurotransmission(Reference Hennebelle, Zhang and Metherel61).
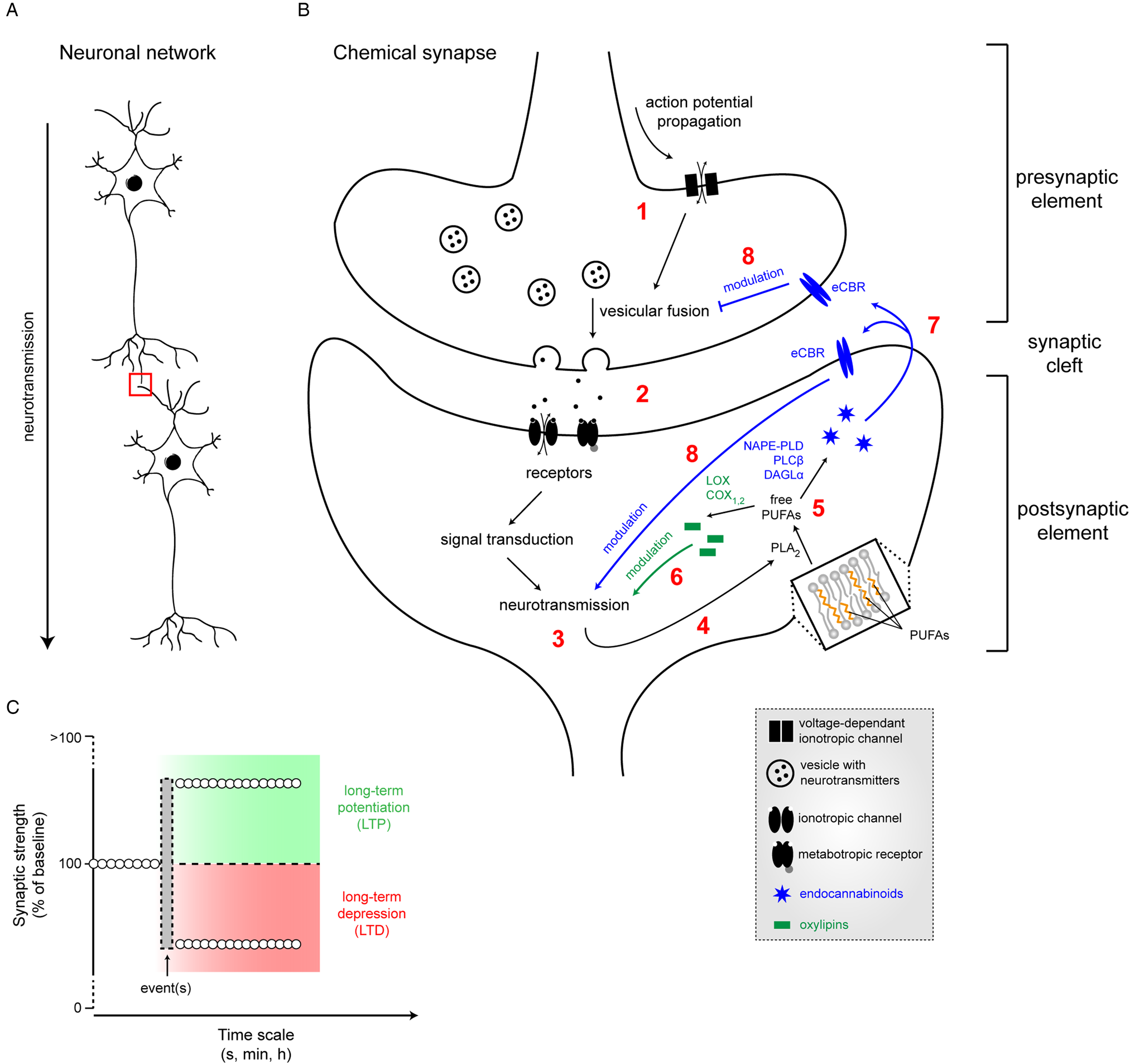
Fig. 3. (Colour online) Neurotransmission and its modulation by PUFA. (A) Neurotransmission is the process by which neurons transmit electric signals in an organised network. (B) The chemical synapse consists of a presynaptic element (axon terminal) in close vicinity to a postsynaptic element (dendritic bouton), separated by the synaptic cleft. Propagation of an action potential (1) leading to the opening of voltage-dependant channels, promoting vesicular fusion. Released neurotransmitters in the synaptic cleft (2) will bind to postsynaptic receptors, inducing neurotransmission (3) through a cascade of events. In turn, the activation of neurotransmission will induce the release of membrane-bound PUFA into cytosolic free PUFA by phospholipase A2 (PLA2) (4). Free PUFA (5) will be converted into either oxylipins, therefore modulating neurotransmission (6) or into endocannabinoids (eCB) that will bind to endocannabinoid (eCBR) receptors (7), thus also modulating neurotransmission (8). (C) Following event(s), synaptic strength can be positively (LTP, long-term potentiation) or negatively modulated (LTD, long-term depression) over different time scales. LOX, lipoxygenase; COX, cyclooxygenase; NAPE-PLD, N-acylphosphatidylethanolamine phospholipase D; DAGL, diacylglycerol lipase-α; PLC, phospholipase C; NMDA, N-methyl D aspartate.
In vitro effect of arachidonic acid, EPA and DHA on neuronal transmission
A few studies investigated the impact of free LC PUFA application on cultured cells and effects differ from one study to the other. In human embryonic kidney cells expressing glutamate transporter-1, applications of DHA at 100 and 200 μm were able to increase and decrease glutamate transport, respectively, an effect that depended upon intracellular calcium(Reference Berry, Hayes and Murphy62). Besides, another study has shown that DHA (3–60 μm) reduced glutamate uptake in rat astrocytes, while ARA had no effect(Reference Grintal, Champeil-Potokar and Lavialle63). In mouse embryonic hippocampal neurons, 1 μm DHA increased spontaneous glutamatergic activity(Reference Cao, Kevala and Kim38). In acutely dissociated rat pyramidal neurons, DHA dose-dependently (15–30 μm) potentiated N-methyl-D-aspartate-induced currents, together with ARA, while oleic acid, an MUFA, had no effect(Reference Nishikawa, Kimura and Akaike64). In Sf-9 cells, both DHA and ARA induced GABAA receptor desensitisation, while oleic acid had no effect(Reference Nabekura, Noguchi and Witt65). Moreover, in dissociated rat substantia nigra neurons, both 5 μm DHA and 15 μm ARA reduced GABAergic currents(Reference Hamano, Nabekura and Nishikawa66). One study investigated the effects of n-3/n-6 PUFA on cholinergic neurotransmission. In Xenopus oocytes expressing acetylcholine receptors, both LA and α-linolenic acid reduced acetylcholine-induced currents when perfused at 10 μm, while these effects were opposed (increased currents) as both compounds washed out(Reference Nishizaki, Ikeuchi and Matsuoka67). Another study on Xenopus oocytes observed increased TRPV1 (vanilloid receptor 1) currents following bath applications of both EPA and DHA (50 μm), an effect due to enhanced voltage-dependent activation of the channel itself(Reference Matta, Miyares and Ahern68). In cultured SH-SY5Y cells, application of free DHA (70 μm), but not ARA (70 μm), during 3 days resulted in increased basal noradrenaline release(Reference Mathieu, Géraldine and Denis69). The lack of consistency between these studies could be explained by several factors: (i) cultured cells are poorly representing the physiology of in vivo neuronal activities, (ii) free PUFA application is not a usual delivery route for PUFA, since they are vastly esterified to phospholipids in brain cell membrane and (iii) delivered PUFA are metabolised into derivatives (oxylipins or eCB) that are active, or not, on synaptic plasticity. As a result, several groups demonstrated the role of PGE2 synthesised from ARA by COX-2 as a retrograde messenger in hippocampal glutamatergic synaptic signalling which facilitates long-term potentiation (LTP) (Fig. 3C) in the hippocampus(Reference Bazan70–Reference Akaneya72).
Ex vivo effect of arachidonic acid, EPA and DHA on neurotransmission
Brain slices are integrated neuronal models to study ex vivo administration of molecules on neuronal transmission. Studies using brain slices have also investigated the role of PUFA through the delivery of free LC PUFA or some of their derivatives. Bath applications of free DHA (50 μm) blocked the induction of long-term depression (LTD) (Fig. 3C) in hippocampal slices(Reference Nabekura, Noguchi and Witt65). Conversely, application of 30 μm DHA resulted in specific alteration of synaptic plasticity in cortico-striatal slices, with facilitation of LTP, but without affecting LTD(Reference Mazzocchi-Jones73). However, another study showed that perfusion of low concentration ARA (10 μM) activates LTP, while DHA and EPA deactivate LTP in the hippocampus(Reference Mirnikjoo, Brown and Kim74), suggesting that the synaptic effects of LC PUFA is dose dependent, or that DHA has a bidirectional activity in the hippocampus(Reference Itokazu, Ikegaya and Nishikawa75). Both ARA and DHA (30 μm) prevented bromoenol lactone (an inhibitor of DHA release)-induced impairment of LTP in rat hippocampi, while 30 μm LA was ineffective(Reference Fujita, Ikegaya and Nishikawa76). ARA effect on neuroplasticity could be mediated by its derivatives. As a result, epoxyeicosatrienoic acid, a non-classic eicosanoid derived from ARA, reduced glutamate release in hippocampal slices by opening G protein-coupled inwardly-rectifying potassium channels(Reference Mule, Orjuela Leon and Falck77). Similarly, N-palmitoylethanolamine, an endogenous compound from the eCB family that derives from hexadecanoic acid, increased spontaneous GABAergic inhibitory post-synaptic current frequencies when applied at 10 μm, an effect that was prevented in the presence of a GPR55 receptor antagonist(Reference Musella, Fresegna and Rizzo78). Again, the variety of the experimental paradigms leads to a large spectrum of results.
In vivo effect of PUFA manipulation on neurotransmission
In vivo, most studies investigated the impact of dietary PUFA changes, even though a study showed that free DHA, administered at 25 nm intracerebroventricularly, decreased the slope of field excitatory post-synaptic potentials (EPSC) within the CA1 region of the hippocampus, while having opposite effects in the dentate gyrus(Reference Itokazu, Ikegaya and Nishikawa75). Some studies also pinpointed that endogenous free DHA or ARA, experimentally modulated by the use of inhibitors of PLA, can regulate LTP(Reference Mazzocchi-Jones73,Reference Fujita, Ikegaya and Nishikawa76) . The modulation of endogenous levels of ARA and DHA by dietary means also allowed to determine whether the endogenous level of PUFA influences neurotransmission and synaptic plasticity. Indeed, dietary supplementations in LC n-3 PUFA (EPA and DHA) resulted in an increased amount of DHA in the brain and, depending on the brain structure, decreased the levels of ARA(Reference Rey, Delpech and Madore79). Conversely, dietary n-3 PUFA deficiency decreases the brain DHA level and, dependent upon brain structures, the age of the animal model used or the starting date of the diet, increases ARA(Reference Lafourcade, Larrieu and Mato39,Reference Thomazeau, Bosch-Bouju and Manzoni40,Reference Delpech, Thomazeau and Madore80–Reference Manduca, Morena and Campolongo83) . In any case, the decreased level of brain DHA triggered by a n-3 PUFA-deficient diet is paralleled by an increase of docosapentaenoic acid (22:5, n-6)(Reference Dyall84). To our knowledge, only one study reported that n-6 docosapentaenoic acid had no effect on neurotransmission in the mouse hippocampus(Reference Taha, Zahid and Epps85).
Developmental n-3 PUFA deprivation also resulted in impaired LTP in the hippocampus of young (P18-24) mice(Reference Cao, Kevala and Kim38,Reference Thomazeau, Bosch-Bouju and Manzoni40) . Moreover, lifelong dietary n-3 PUFA deficiency in mice (starting at gestation) impaired LTD in both prefrontal cortex and nucleus accumbens synapses at adulthood, an effect explained by the dissociation of the Gi/o protein to cannabinoid receptor 1 (CB1R)(Reference Lafourcade, Larrieu and Mato39). In Caenorhabditis elegans lacking Δ-6-desaturase, the enzyme responsible for LC PUFA synthesis, reduced evoked excitatory post-synaptic current amplitudes were observed, explained by partially depleted vesicles(Reference Lesa, Palfreyman and Hall86). Dietary n-3 PUFA deficiency not only disturbs neurotransmission but also sensitises to exogenous stimuli or ageing. As a result, mice fed for 2 months with a n-3 PUFA-deficient diet starting at weaning, displayed impaired LTD following acute inflammation, an effect not observed in mice fed with a control diet(Reference Delpech, Thomazeau and Madore80). Age-related glutamatergic dysfunction is worsen by dietary n-3 PUFA deficiency(Reference Latour, Grintal and Champeil-Potokar87). Furthermore, in the hippocampus, n-3 PUFA dietary supplementation with DHA rescued the alteration of both LTD and LTP induced by prenatal ethanol consumption(Reference Patten, Brocardo and Christie88). Moreover, both n-3 and n-6 PUFA dietary supplementation induced stronger LTP in aged rats, compared to a control diet(Reference Kashiyae, Kontani and Kawashima89). DHA effect on glutamatergic neurotransmission could be indirect, through the regulation of glutamate transport in astrocytes(Reference Grintal, Champeil-Potokar and Lavialle63).
Research examining the impact of dietary PUFA manipulation on neurotransmission has largely focused on the dopaminergic system. In rats, strong evidence now suggests that dopaminergic transmission can be modulated by dietary n-6/n-3 PUFA. In fact, dietary n-3 PUFA deficiency (during gestation until adulthood) decreased dopamine release in cortical structures, together with increased dopamine metabolites(Reference Zimmer, Hembert and Durand90–Reference Kodas, Vancassel and Lejeune93). Moreover, electron microscopy revealed fewer dopamine vesicles and reduced vesicle pool(Reference Zimmer, Delion-Vancassel and Durand91,Reference Zimmer, Delpal and Guilloteau94) . In the hippocampus, depletion of vesicles was also observed, together with decreased staining of tyrosine hydroxylase (the limiting enzyme for dopamine synthesis), decreased vesicular transporter of monoamines (vesicular monoamine transporter 2), combined with an increased expression of both dopamine D1 and D2 receptors as a result of decreased dopamine availability(Reference Kuperstein, Yakubov and Dinerman95,Reference Kuperstein, Eilam and Yavin96) . In fact, a lower number of tyrosine hydroxylase-positive cells were also found in the two midbrain dopaminergic nuclei, the substantia nigra pars compacta and the ventral tegmental area(Reference Ahmad, Park and Radel97).
Besides dopaminergic systems, other neuromodulatory systems are also sensitive to dietary PUFA manipulation. Indeed, a study observed that n-6 PUFA-rich diets reduced serotonin transporters in the hippocampus(Reference du Bois, Deng and Bell98), while others showed that dietary supplementation with LC PUFA (ARA and DHA) can restore the decreased dopamine and serotonin levels in frontal regions induced by a developmental deficiency in PUFA precursors (α-linolenic acid and LA)(Reference de la Presa Owens and Innis99). Muscarinic acetylcholine receptors can also be downregulated following dietary n-3 PUFA deficiency(Reference Aïd, Vancassel and Poumès-Ballihaut100) and diets high in n-6 PUFA(Reference du Bois, Bell and Deng101). These observations are paralleled with a study showing that DHA supplementation could restore age-related decline of acetylcholine levels(Reference Favrelière, Perault and Huguet102). In 8-week old adult mice, downregulation of several genes involved in GABAergic neurotransmission were found following n-3 PUFA deprivation, starting at gestation until weaning(Reference Maekawa, Watanabe and Iwayama103).
Opposite to n-3 PUFA deficiency, LC n-3 PUFA dietary supplementation has also been investigated. In rats, dietary supplementation from gestation until adulthood with fish oil (rich in EPA and DHA) reduced monoamine oxidase B activity in cortical regions, which led to increased dopamine levels and higher dopamine D2 receptor binding(Reference Chalon, Delion-Vancassel and Belzung104). In stressed rats, n-3 PUFA supplementation with DHA and EPA was able to restore inhibitory post-synaptic currents in the hippocampus, together with the restoration of GABA release, which was correlated with improved spatial memory(Reference Pérez, Peñaloza-Sancho and Ahumada105). Following a 10-month dietary supplementation with DHA in mice, entorhinal cortex neurons presented significantly increased spontaneous excitatory post-synaptic current frequencies, associated with increased conductance and capacitance(Reference Arsenault, Julien and Chen106). Finally, n-3 PUFA supplementation with DHA in Long-Evans rats, starting at adulthood and lasting for 3 months, increased serotonin and norepinephrine levels in both the prefrontal cortex and the nucleus accumbens(Reference Olsson, Shoaf and Salem107). Altogether, these in vivo manipulations of LC PUFA show that n-3 PUFA are critical for proper synapse homoeostasis and can rescue synapse function under some pathological conditions.
Possible mechanisms by which PUFA can modulate synaptic transmission
Several mechanisms can explain how PUFA influence synaptic function. First, as structural elements of plasmic membranes, PUFA can modulate membrane dynamics and thus the function and traffic of transmembrane proteins, as well as membrane-associated proteins. These proteins are legion at both pre- and post-synaptic elements (receptors, transporters, ion channels, etc.), because of their essential functions in the synapse. Secondly, as previously mentioned, free PUFA are converted to bioactive mediators, oxylipins, which act in the modulation of neuroinflammation has been documented(Reference Layé, Nadjar and Joffre57) and are potential modulators of neurotransmission(Reference Bazan70). This mode of action of PUFA is complex and remains poorly understood, therefore being an intense research field. Thirdly, PUFA are precursors of eCB, which are lipid mediators with essential functions in neurotransmission and synaptic plasticity. These three aspects are developed hereafter.
Membrane effects
When associated to phospholipids, PUFA play an important role in the structure of membranes, by determining the curvature and flexibility of the bilayer (Fig. 4). After being considered as homogeneous and inert media, it is now clear that membranes organise in both highly structured and dynamic micro-domains, which greatly influence the functions of transmembrane proteins. Membrane fluidity is highly determined by its fatty acid composition(Reference Mansilla, Cybulski and Albanesi108–Reference Pinot, Vanni and Pagnotta110). In the brain, membranes are rich in PUFA, usually DHA and ARA, compared to other tissues(Reference Bazinet and Layé4). For instance, while phospholipids usually contain none or one PUFA, most phospholipids in the brain contain two PUFA(Reference Marszalek and Lodish111,Reference Takamori, Holt and Stenius112) . In the brain, DHA is mostly found in ethanolamine plasmalogen, phosphatidylserine and phosphatidylethanolamine, these two phospholipids being densely found in the inner leaflet of the phospholipidic bilayer, while ARA is mainly esterified in phosphatidylcholine(Reference Wassall and Stillwell113).

Fig. 4. (Colour online) Influence of PUFA on membrane organisation. The fluid membrane is composed of several PUFA. Lipid rafts are subdomains rich in cholesterol while DHA-rich domains allows for membrane flexibility, due to their leakiness. Microdomains can dynamically reorganise into macrodomains.
DHA has more flexibility than ARA, suggesting that the beneficial effects of DHA in brain functions are partly due to its action on membrane biophysical properties(Reference Wassall and Stillwell113). Attention has been recently focused on lipid rafts, which contain both sphingolipid- and cholesterol-rich domains(Reference Lingwood and Simons114). It has been hypothesised that synapses are especially enriched in lipid rafts and that these micro-domains are necessary for protein trafficking, signalling pathways and synapse maintenance(Reference Hering, Lin and Sheng115). However, DHA-rich domains are functionally and organisationally the opposite of lipid rafts, because of their ‘leaky’, dynamic, thin and flexible domains(Reference Wassall and Stillwell113). High-density lipid rafts and low-density DHA domains are permanently competing. It is suggested that lipid rafts are initially small nano-domains that organise together to subsequently form greater domains, of the microscale (Fig. 4). In this configuration, the organisation of lipid rafts would be controlled by DHA, which aggregates nano-domains together or conversely disrupt large lipid rafts(Reference Wassall and Stillwell113,Reference Shaikh116,Reference Wassall and Stillwell117) . These dynamic changes of membrane biophysics induce a modification in the lipid environment of transmembrane proteins, which ultimately controls their function and trafficking. The functional significance of those effects is that receptors, transporters and ion channels could be modulated by different levels of DHA present at the synaptic membrane. This way, DHA would be a potent modulator of synapse function. This is illustrated in the context of treatments for depressive disorders. Some clinical studies suggested that DHA and/or EPA supplementation can improve the efficacy of classical antidepressant treatments, targeting serotonergic receptors(Reference Grosso, Galvano and Marventano118,Reference McNamara, Strimpfel and Jandacek119) . Evidence suggests that n-3 PUFA could disrupt lipid rafts, which would improve the signalling pathway of G protein-coupled receptors (GPCR), including serotonergic receptors, explaining the higher efficacy of antidepressants(Reference Czysz and Rasenick120). More generally, determining which synaptic proteins need lipid rafts or DHA-domains as optimal lipid environments would be a first step to understand the role of DHA in synaptic membrane dynamics.
Action of free PUFA on ion channels and ionotropic receptors in the brain
As excitable cells, neurons express voltage-gated ion channels that are responsible for action potential initiation/propagation, neurotransmitter release and post-synaptic depolarisation/hyperpolarisation. Modulation of voltage-gated ion channels is therefore crucial to maintain homoeostasis of neurotransmission(Reference Frank121), on both pre- and post-synaptic poles. It is now well established that PUFA such as DHA or ARA modulate these channels(Reference Elinder and Liin122,Reference Poling, Vicini and Rogawski123) .
The impact of PUFA on ion channels has been initially investigated in another type of excitable cell, cardiomyocytes (in the heart), since both DHA and EPA have the potential to prevent and stop arrhythmias(Reference Leaf124–Reference Leaf, Xiao and Kang126). Application of free DHA on isolated cardiomyocytes decreases their excitability by decreasing currents from sodium and calcium voltage-gated channels(Reference Leaf124). In the majority of cases, PUFA are decreasing the activity of voltage-gated ion channels by shifting leftwards the threshold of their inactivation door, making these channels inactivated quicker and at lower hyperpolarised voltages. DHA, EPA and ARA are the most studied PUFA for this purpose and they generally work in the same direction, but DHA tend to have a stronger impact on channel activity(Reference Poling, Vicini and Rogawski123,Reference Leaf124) . However, since these studies applied free PUFA, which is not representative of physiological PUFA levels, one must remain cautious about their interpretations.
In the brain, similar effects of PUFA on ion channels have been observed. Application of either DHA or EPA is able to decrease the excitability of neurons by shifting the inactivation current of both sodium and calcium channels(Reference Vreugdenhil, Bruehl and Voskuyl127,Reference Xiao and Li128) . PUFA also modulate ionotropic GABA and glutamate receptors. Direct application of free DHA has been shown to increase currents from GABAA receptors(Reference Nabekura, Noguchi and Witt65,Reference Taha, Zahid and Epps85,Reference Søgaard, Werge and Bertelsen129) , increase opening probability of N-methyl-D-aspartate channels(Reference Nishikawa, Kimura and Akaike64) and decrease currents from α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors(Reference Wilding, Chai and Huettner130,Reference Wilding, Zhou and Huettner131) . As a consequence, modulatory effects of PUFA on neuronal excitability can lead to decreased excitatory sharp waves occurring in epilepsy episodes, as demonstrated with 100 μm free DHA application on hippocampal slices(Reference Taha, Zahid and Epps85).
Several hypotheses exist in the literature to explain the different effects of PUFA on ion channels. It was initially proposed that PUFA directly bind to channels, inducing conformational changes, thus modifying channel properties(Reference Hamano, Nabekura and Nishikawa66,Reference Poling, Vicini and Rogawski123,Reference Vreugdenhil, Bruehl and Voskuyl127) . It has also been proposed that PUFA could act on the extracellular side of the membrane, based on results showing that pre-treatment of cardiomyocytes with bath application of delipidated serum albumin counteracted PUFA-induced antiarrhythmic effects(Reference Leaf124). However, PUFA can easily reach the inner leaflet of the plasma membrane by ‘flip-flop’ mechanisms(Reference Hamilton132,Reference Liu, Green and Mann133) , disabling therefore the drawing of any clear conclusion. Some studies have hypothesised that PUFA could modulate ion channels at the synapse by modifying membrane fluidity. This has been observed by showing that effects of DHA on GABAA currents can be mimicked using agents that change membrane fluidity(Reference Søgaard, Werge and Bertelsen129). This reinforces the fact that ion channels are sensitive to their lipid surroundings(Reference Maguy, Hebert and Nattel134).
Besides, several studies suggested that PUFA modulate the function of ion channels via lipoelectric mechanisms(Reference Elinder and Liin122,Reference Boland and Drzewiecki135–Reference Tigerholm, Börjesson and Lundberg139) . Indeed, the negative charges of PUFA act as stabilisers of the positive charge of the voltage sensor from voltage-gated ion channels. Here, both the flexibility of the double bonds and the length of the PUFA carbon chain allow the ion channel to adapt its conformational shape. These characteristics could explain why DHA is the PUFA showing the best modulatory activity, as compared to other PUFA.
Importantly, in all of these studies, application of free PUFA in the medium induced effects on very short-term timescales, within seconds or minutes(Reference Taha, Zahid and Epps85,Reference Vreugdenhil, Bruehl and Voskuyl127,Reference Xiao and Li128,Reference Danthi, Enyeart and Enyeart140) . Moreover, trials with non-metabolisable analogues (such as eicosatetraynoic acid for ARA) did not reproduce the effects of free PUFA(Reference Kehl141), but potently blocked fast-inactivating potassium currents(Reference Meves142). Moreover, the inhibition of PUFA metabolites did not prevent the effect of free PUFA application(Reference Nishikawa, Kimura and Akaike64). This suggests that ion channels might be modulated by PUFA themselves, whilst being free or when inserted at the membrane. However, it remains unclear whether free PUFA are released under physiological conditions to modulate ion channels at the synapse. As a clue, it has been demonstrated that synaptic transmission can trigger the release of PUFA. In particular, a few receptors, such as N-methyl-D-aspartate receptors(Reference Dumuis, Sebben and Haynes143,Reference Tapia-Arancibia, Rage and Récasens144) and 5 hydroxytryptaminereceptors 2A(Reference Kurrasch-Orbaugh, Parrish and Watts145), are able to activate phospholipases, which may then release ARA or DHA. This hypothesis was reviewed in 2009(Reference Rosa and Rapoport146). Upon release, the majority of PUFA are metabolised into PUFA derivatives (see later), but free ARA can also be involved, by itself, in the modulation of ion channels and synapse activities, especially during short-term potentiation and LTP(Reference Carta, Lanore and Rebola147–Reference Wolf, Izumi and Zorumski151).
Effects of PUFA on G protein-coupled receptor
GPCR signalling pathways are highly involved in the function of the synapse. The role of PUFA on GPCR has been extensively studied in the retina, since DHA can represent up to 60 % of total fatty acids within the photoreceptor membranes, which contain the GPCR rhodopsin(Reference Fliesler and Anderson152). Early studies have shown that DHA-rich photoreceptor membranes can influence photon capture by changing the spatial conformation of rhodopsin in response to light absorption(Reference Litman, Niu and Polozova153). As a matter of fact, the activation kinetic of rhodopsin was proportional to the number of double bonds found within membrane phospholipids(Reference Litman, Niu and Polozova153). A recent study in Drosophila, reared under PUFA-deficient diets (devoid of all PUFA), has shown that photoreceptors are two to three times slower to respond to light stimuli than flies reared under standard diets(Reference Randall, Liu and Chu154). In parallel, extracellular PUFA application potentiated light-activated currents(Reference Chyb, Raghu and Hardie155,Reference Delgado and Bacigalupo156) in ommatidia (compound unit in invertebrates) and in dissociated rhabdomeres (rodlike structure within ommatidia). A review has detailed all evidence regarding n-3 PUFA and retinal physiology(Reference Acar157). Since rhodopsin receptors belong to a vast GPCR superfamily(Reference Katritch, Cherezov and Stevens158), these studies are useful to understand the effect of PUFA on brain GPCR. In the brain, dopamine, serotonin and eCB receptors are GPCR of the same super family, that are modulated by PUFA, as previously mentioned. The precise mechanism of action of PUFA on these receptors is still not clear, but current knowledge is driven by PUFA–rhodopsin interactions. Interestingly, it has been observed that GPR40 and GPR120 are activated by free PUFA binding, especially medium to LC PUFA(Reference Czysz and Rasenick120,Reference Briscoe, Tadayyon and Andrews159) .
Potential of PUFA derivatives on neurotransmission
Finally, it is important to consider the role of PUFA derivatives (oxylipins and eCB) on neurotransmission (Fig. 3B). COX-1 and 2 are two key enzymes that convert ARA into prostaglandins. A study has shown that COX-2, but not COX-1 inhibitors, greatly reduced post-synaptic membrane excitability and LTP induction in hippocampal neurons(Reference Chen, Magee and Bazan160). These effects were rescued by bath applications of PGE2 at 2 μm(Reference Chen, Magee and Bazan160). Platelet-activating factor (PAF) derives from ARA and is synthesised from phosphatidylcholine by PLA2(Reference Murakami, Nakatani and Atsumi161). In cultured neurons, application of non-hydrolysable PAF increased the frequency of miniature excitatory post-synaptic currents as well as the excitatory synaptic transmission mediated by glutamate(Reference Clark, Happel and Zorumski162). These findings were later confirmed when antagonism of PAF receptors prevented the induction of LTP in the CA1 region(Reference Kato, Clark and Bazan163), while PAF itself induced LTP(Reference Wieraszko, Li and Kornecki164). Moreover, in PAF-deficient mice, LTP induced by high frequency stimulation (eight trains, each of eight pulses at 200 Hz) is attenuated compared to control mice(Reference Chen, Magee and Marcheselli165). These results suggest a potential role of PAF as a retrograde messenger, which appeared crucial in the maintenance of LTP in the hippocampus, but further studies are needed to understand the underlying mechanism.
Other important PUFA derivatives involved in neurotransmission are eCB, which bind to CB1R and CB2R GPCR. CB1R is abundant and widely distributed in the brain, while the expression of CB2R is more restricted to the immune system. eCB are lipid mediators that act retrogradely or anterogradely at the synapse. Anandamide (AEA) and 2-arachidonoylglycerol are the two main eCB, derived from the n-6 PUFA ARA(Reference Di Marzo, Stella and Zimmer166,Reference Di Marzo167) . AEA biosynthesis occurs at the pre- and post-synapse by the action of N-acylphosphatidylethanolamine phospholipase D. AEA is degraded post-synaptically by fatty acid amide hydrolase. As a result, AEA acts as an anterograde signal acting at postsynaptic targets, or as an intracellular mediator. 2-arachidonoylglycerol acts as a retrograde signal. It is therefore biosynthesised post-synaptically by diacylglycerol lipase-α while degraded pre-synaptically by monoacylglycerol lipase. DHA is also converted into DHEA, known to promote neurite outgrowth and synaptogenesis as previously described(Reference Kim and Spector168). However, its role in the regulation of neurotransmission and synaptic plasticity has been poorly described. AEA, DHEA and 2-arachidonoylglycerol are parts of larger families of lipids, N-acylethanolamines and 2-acylglycerols, respectively(Reference Bosch-Bouju and Layé60,Reference Di Marzo169) .
At the cellular level, synaptic plasticity is the main functional outcome of the eCB system in the brain. Indeed, eCB reduce synaptic efficacy, over very short, medium or long time scales, depending on the signalling pathways that are triggered by eCB production(Reference Chevaleyre, Heifets and Kaeser170–Reference Cachope172). eCB are produced on-demand and released to activate receptors, leading to a decrease of synaptic transmission efficiency(Reference Chevaleyre, Heifets and Kaeser170). eCB are rapidly degraded and released PUFA are re-esterified at the membrane, to precisely regulate duration of eCB action(Reference Viveros, Marco and Llorente171).
Importantly, endogenous levels of eCB mediators can be modulated by changes in dietary n-6:n-3 PUFA ratio(Reference Cachope172–Reference Piomelli, Astarita and Rapaka174). Our laboratory precisely investigated the impact of dietary PUFA on the eCB-dependent synaptic plasticity. We demonstrated that developmental dietary n-3 PUFA deficiency abolishes the eCB-dependent synaptic plasticity in both the prefrontal cortex and the nucleus accumbens, combined with the uncoupling of CB1R from their effectors Gi/o proteins(Reference Lafourcade, Larrieu and Mato39). To our knowledge, this is the first evidence that a change in dietary precursors can have a strong impact on the eCB system. The consequences of developmental dietary n-3 PUFA deficiency on eCB-dependent synaptic plasticity has also been shown in the hippocampus. We demonstrated that n-3 PUFA deficiency strongly impaired the eCB-dependent plasticity at GABAergic synapses, which prevents the induction of plasticity at glutamatergic synapses(Reference Lafourcade, Larrieu and Mato39,Reference Thomazeau, Bosch-Bouju and Manzoni40,Reference Manduca, Morena and Campolongo83) .
In parallel, one study reported that DHA supplementation increased levels of CB1R and TRPV1, in terms of mRNA expression and protein levels(Reference Berger, Crozier and Bisogno175), while DHEA enhanced glutamatergic neurotransmission more potently than DHA(Reference Artmann, Petersen and Hellgren176). Our results on CB1R desensitisation(Reference Lafourcade, Larrieu and Mato39,Reference Wood, Williams and Pandarinathan177) have been reinforced by a study showing that a krill oil-based diet (rich in both EPA and DHA), given for 6 weeks to adult mice, enhanced the activity of CB1R(Reference Pan, Zhang and Wei-Wang178). It is now known that CB1R can be easily desensitised and internalised following ligand binding(Reference Kim, Spector and Xiong179). This has been particularly studied in the context of chronic cannabinoid consumption. The mechanisms of desensitisation and downregulation are not completely elucidated, but they likely involve the phosphorylation of these receptors and transcription of immediate early genes(Reference Larrieu, Madore and Joffre180,Reference Yamada, Takeo and Koppensteiner181) . Studies on the role of dietary PUFA on CB1R demonstrate that dietary PUFA can constitute another powerful mechanism for the regulation of the functionality of CB1R.
Conclusion and future directions: PUFA as important modulators of synapse homoeostasis, implications for synaptopathies?
Investigations have demonstrated so far that LC PUFA can efficiently modulate synaptic transmission by acting on membrane fluidity and organisation, on voltage-gated ion channels/receptors as well as through derivatives such as oxylipins or eCB (Fig. 5). These synaptic targets of LC PUFA are in close interaction with each other since membrane fluidity strongly impacts the activity of voltage-gated ion channels and all transmembrane proteins(Reference Maguy, Hebert and Nattel134), while eCB can modulate ion channels via receptor-independent mechanisms(Reference Martini, Waldhoer and Pusch182). These intertwined mechanisms partly explain the high variety of results found in the literature and the lack, so far, of a clear picture. However, the common feature of these overlapping mechanisms is that PUFA appear as major actors of metaplasticity, which encompasses all events that can modify the ability of a neuron or a network to induce plasticity(Reference Lazenka, Selley and Sim-Selley183–Reference Abraham and Bear186). Dietary PUFA can induce long-term modifications of neuronal excitability and network homoeostasis. In addition, eCB are major actors of metaplasticity since they can prime LTP of excitatory synapses by silencing inhibitory ones(Reference Abraham187,Reference Hulme, Jones and Abraham188) . New emerging modulators of neurotransmission are bioactive oxylipins, formed from different PUFA, whose functions in physiology is still intensely studied(Reference Hulme, Jones and Raymond189). In this context, PUFA are thus good candidates to participate to metaplasticity, revealing that diet, as much as life experiences, can influence synapse homoeostasis and thus brain states.
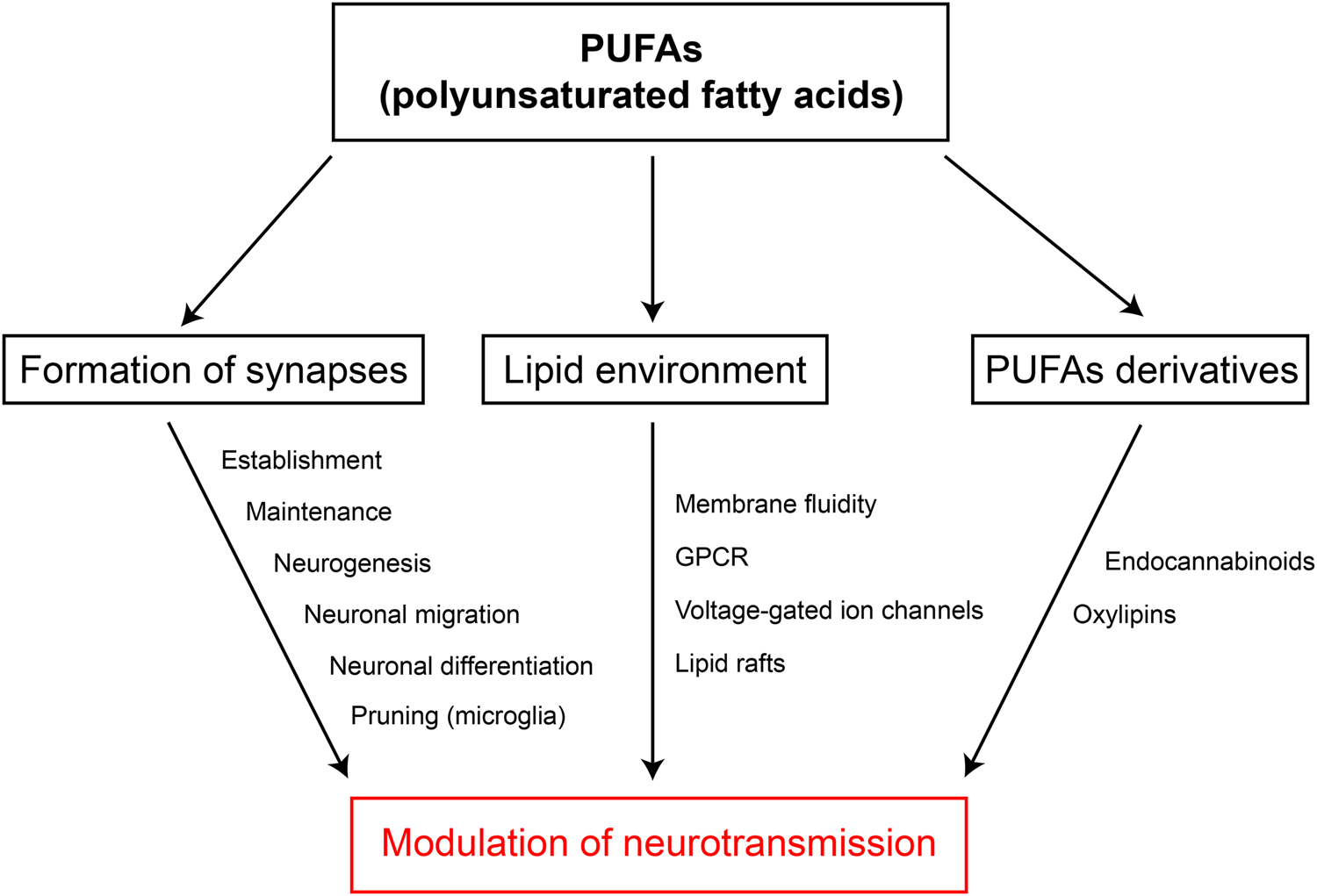
Fig. 5. (Colour online) Modulation of neurotransmission by PUFA. Summary of the evidence showing how PUFA can modulate neurotransmission. GPCR, G protein-coupled receptors.
In parallel to experimental studies, clinical trials are investigating the potential of LC PUFA, in particular n-3 PUFA supplementation, in the treatment of brain disorders. Due to their wide impacts on synapse function, potential pharmacological applications of PUFA to treat synaptopathies in the clinic could include neurodevelopmental and neurodegenerative diseases, neuropsychiatric disorders as well as brain traumas, such as strokes and epilepsy. In this perspective, understanding the mechanisms by which PUFA exert their synaptic effects appears essential.
Financial Support
This study was supported by the Fondation pour la Recherche Médicale (DEQ20170336724), the Région Nouvelle Aquitaine (2015-1R30116), the Fondation de France (00070700), the Société Française de Nutrition, as well as the Brain & Behavior Research Foundation (NARSAD 25083).
Conflict of Interest
None.
Authorship
All authors were involved in all aspects of preparation of this paper.