CVD has a high prevalence and huge burden worldwide, and has become one of the leading causes of human death, and 80 % of CVD takes place in the developing world(Reference Zhou, Wang and Zhu1). Numerous factors are related to CVD, of which circulating homocysteine (Hcy) and folate levels are the focus of much attention in recent years. Related research shows that folate deficiency and elevated Hcy levels are associated with human health, CVD and mortality(Reference Veeranna, Zalawadiya and Niraj2–Reference Zhong, Xu and Xu4). Hcy can also be used to predict diabetes, dementia and pregnancy disorders(Reference Xu, Wu and Liu5–Reference Boyama, Cepni and Imamoglu7). Folate deficiency is also associated with chronic diseases such as CVD, cancer and cognitive dysfunction; maternal folate nutritional status is related to the risk of neural tube defects in the offspring(Reference Ebara8). Folate is also one of the main determinants of circulating Hcy(Reference Zappacosta, Mastroiacovo and Persichilli9). Folate supplementation may prevent CVD and some cancers and lower circulating Hcy(Reference Anderson, Jee and Charleston10).
For this purpose, some developed countries have carried out several large-scale population-based surveys to evaluate circulating Hcy and folate status and taken evidence-based actions such as folate fortification(Reference Refsum, Nurk and Smith11). They have established policies for folate fortification of cereals and flour to decrease the prevalence of hyperhomocysteinaemia (HHcy), and observed some plausible changes including in CVD prevention(Reference Jacques, Selhub and Bostom12–Reference Tucker, Mahnken and Wilson14), which is of valuable reference for developing countries. Folate is a vitamin that cannot be synthesised by human body and only obtained from microbes that break down food during digestion(15). Therefore, folate and Hcy levels vary widely due to high geographical, lifestyle, dietary and racial/ethnic diversity(Reference Monteagudo, Scander and Nilsen16–Reference Calcaterra, Larizza and De Giuseppe18). Accurate estimates of the prevalence of a condition and relevant determinants are essential as a source of primary information and for rational planning of health services, and would allow public health policymakers to assign sufficient priority and resources to its management and prevention(Reference Kearney, Whelton and Reynolds19), which is also the same for folate deficiency and HHcy, especially in resource-constricted areas and developing countries. Nonetheless, there is a paucity of equivalent data from developing countries.
Based on previous studies, the prevalence of HHcy in Chinese population is 27·5 %(Reference Yang, Fan and Zhi20 ), and the prevalence and the risk factors may also vary due to geographical, ethnic, social and dietary diversity(Reference Yang, Fan and Zhi20). Xinjiang, a multi-ethnic province with a population of various lifestyles and dietary habits living in urban, agricultural and stock-raising regions(Reference Zhou, Zhao and Heizhati21), is located in northwest China and Central Asia and provides an ideal setting for this type of study, where folate fortification is not mandatory as well. Local nomads have been residing in stock-raising areas like villages, forests and mountains; move around depending on seasonal changes; and spend most of their time in remote areas, and thus their living is characterised by much dependence on animal products, including animal oil, meat, milk and dairy products, and by limited access to fresh fruit and vegetables(Reference Zhou, Zhao and Heizhati21,Reference Han, Hu and Tang22) . Only an existing study conducted in rural Xinjiang showed a high prevalence of HHcy, but failed to analyse the effects of different living environments and folate concentrations on Hcy(Reference Guo, Pang and Guo23).
In countries where folate fortification is not mandatory and where resource is constricted, it is of significant value to identify the target population that would most benefit from folate supplementation. Therefore, the main purpose of the current study was to comprehend folate and Hcy levels and their potential determinants in agricultural, stock-raising and urban regions using a population-based, cross-sectional survey, and to provide clues to other regions with approximate conditions.
Subjects and methods
Site
Emin county, northern Xinjiang, is home to >133 000 people aged ≥15 years from urban, agricultural and stock-raising regions, which makes it an ideal setting for a study of the effects of different lifestyles on Hcy and folate metabolism. The survey was conducted from January to December 2014.
Study population
Multistage stratified random sampling was used to obtain a sample of the population aged ≥15 years. At the first stage, the whole county was divided into three regions as urban, agricultural and stock-raising regions, based on the urban and rural area code issued by the National Bureau of Statistics of the People’s Republic of China, as well as on the regional production mode and the main source of economic income. At the second stage, two townships were randomly selected in each region using simple random sampling. At the third stage, two villages were randomly selected as survey villages in each of the extracted townships. In the final stage of sampling, a given number of participants from each site were selected from communities or villages using lists compiled from local government registers of households. Inclusion criteria encompassed: (i) local inhabitants aged ≥15 years; (ii) residing at current address for ≥6 months; (iii) agreeing to participate and sign an informed consent form. Exclusion criteria included renal insufficiency; pernicious anaemia; malignant tumours; pregnancy; and taking anticonvulsants, anti-tuberculosis drugs, birth control pills, dopamine drugs, folate and/or vitamin B12 supplementation in the past month.
Data collection
Questionnaire, physical examination and biochemical examination
Population health behaviour questionnaires and physical examinations were conducted using onsite surveys to collect detailed information from all participants via a face-to-face interview by trained investigators, which included demographic characteristics (name, gender, age, ethnicity and current address), socioeconomic status (educational status), lifestyle risk factors (cigarette consumption and alcohol intake) and individual and family medical history. Physical examination included measurements of height, body weight, waist circumference (WC) and blood pressure (BP). Laboratory examination included measurement of plasma Hcy and folate.
Measurement of blood pressure, height, weight and waist circumference
BP was presented as the mean of three measurements using an Omron HEM-1000 electronic sphygmomanometer via a standardised procedure(Reference Perloff, Grim and Flack24). All participants were advised to avoid cigarette smoking, alcohol, caffeinated beverages, tea and exercise for at least 30 min prior to measurements. Three BP measurements were taken, after a rest of at least 5 min, from the unclothed right arm of the person in a sitting position at an interval of at least 1 min. Body weight, height and WC were measured using standard methods(25). Height and weight were measured to the nearest 0·1 cm and 0·1 kg, respectively, with the participants in lightweight clothing and without shoes. WC was measured at the midpoint between the lower rib and upper margin of the iliac crest to the nearest 0·1 cm at the end of a normal expiration. BMI was calculated by dividing weight by height-squared (kg/m2).
Laboratory measurements
Venous blood samples were obtained by the same trained nurses in the morning after overnight fasting. Plasma was separated within 30 min of collection by centrifugation (4°C, 20 min, at 1509·3 g ); stored at onsite refrigerators temporarily; transported in a cooler to the clinical laboratory at the People’s Hospital of Xinjiang Uygur Autonomous Region; and stored at –80°C until assayed. Plasma Hcy and folate were measured by the same staff blinded to the aim and design of the study. Plasma Hcy was measured by an enzymatic cycling assay method using a DIRUI equipment of the type CS-T300, with a plasma Hcy precision of 0·1 μmol/l. The kit purchased from Shenzhen Tailed Medical Co. (batch number 20190104) was used. Folate was measured by chemiluminescence method with an UniCel D×I 800 automatic biochemical analyser (Beckman Coulter), with a plasma folate precision of 0·01 ng/ml and with the detectable concentration ranging from 1·0 to 24·2 ng/ml. The folate assay kit was purchased from Beckman Coulter, Inc. (batch number 832212). All measurements were performed in strict accordance with the instrument and reagent instructions.
Diagnostic criteria
HHcy is defined as circulating Hcy ≥10 µmol/l(Reference Sacco, Adams and Albers26). Folate deficiency is defined as a concentration <3 ng/ml(Reference Jacques, Selhub and Bostom12). Hypertension is defined as systolic BP ≥140 mmHg, and/or diastolic BP ≥90 mmHg, and/or use of antihypertensive medication within previous 2 weeks. Overweight and obesity is defined as BMI ≥25 kg/m2 according to WHO classifications(Reference Zhao, Wang and Mu27). Abdominal obesity is defined as WC ≥90 cm for men and ≥85 cm for women. Cigarette consumption is defined as cumulative smoking in the past reaching more than twenty packs of cigarettes and is currently smoking. Alcohol intake is defined as at least once a week within the previous 30 d of the survey.
Statistical analysis
Continuous variables, if normally distributed, were presented as means ± sd and analysed using t test or ANOVA, followed by post hoc analysis (least significant difference test). Categorical variables were expressed as proportion (%) and frequency (n) and analysed using the χ2 test. Hcy and folate levels were compared by covariance analysis among and between groups after adjusting for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, education and ethnicity. Partial correlation analysis was performed between Hcy and folic acid. Univariate logistic regression analysis was performed to select the potential factors that have been speculated to influence Hcy and folate concentrations with P < 0·1 to enter the equation (data not reported). Multivariable logistic regression was performed for Hcy after adjusting for significant variables in univariate regression analysis such as gender, age, BMI, alcohol intake, cigarette consumption, hypertension, folate tertile and regions; and for folate deficiency by adjusting for gender, age, ethnicity, education and regions. OR and 95 % CI were calculated. Tertile of folate was used in the multivariable logistic regression analysis. A P value <0·05 was considered statistically significant. All statistical analyses were performed with SPSS, version 23.0.
Results
Characteristics of the study populations
A total of 1946 participants volunteered to complete the study, of whom 1934 provided blood samples. An additional eight subjects were excluded from the analysis because of insufficient blood sample. Finally, data of 1926 participants with 885 (45·9 %) from urban, 861 (44·7 %) from agricultural and 180 (9·4 %) from stock-raising regions were evaluated. The proportion of subjects enrolled is approximate to that of the local population(28).
As in Table 1, study populations from urban, agricultural and stock-raising regions were similar in terms of age and gender. The prevalence of hypertension, systolic BP and diastolic BP levels, education attainment, cigarette consumption, alcohol intake, overweight and obesity proportion, and ethnicity were significantly different among the three regions (P for all <0·05). In the stock-raising region, Kazakh subjects accounted for 79·1 %, and the prevalence of hypertension was higher compared with agricultural and urban regions (38·3 v. 24·8 v. 18·5 %, P < 0·001). In the urban region, the majority was Han ethnicity, accounting for 63 %, and the proportion of cigarette consumers, alcohol intake and overweight and obese subjects were significantly higher than in rural and pastoral regions (P for all <0·05). In the agricultural region, the majority was also Han ethnicity, accounting for 56·2 %, and the prevalence of hypertension was lower than in urban and stock-raising regions (18·5 v. 24·8 v. 38·3 %, P < 0·001).
Table 1 Characteristics of the study population*
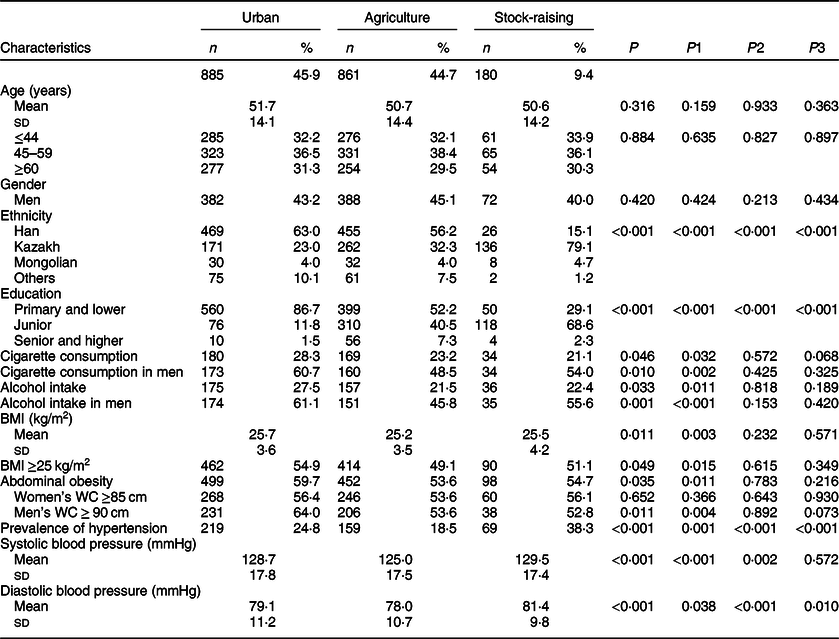
WC, waist circumference.
* We conducted among-group and between-group comparisons using ANOVA followed by post hoc analysis (least significant difference test) and χ2 test and provided specific P-values as P for among-group comparison and as P1 (urban v. agriculture), P2 (agriculture v. stock-raising) and P3 (stock-raising v. urban) for between-group comparisons.
Level of homocysteine and folate
As shown in Table 2, after adjusting for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, education and ethnicity, the folate level of subjects from the stock-raising region was the lowest, followed by those from the agricultural region, and the highest in those from the urban region with statistical significance (3·48 v. 6·50 v. 7·12 ng/ml, P < 0·001). Nonetheless, the mean concentration of plasma Hcy showed no significant differences among subjects from different regions.
Table 2 Homocysteine and folate levels in different populations*
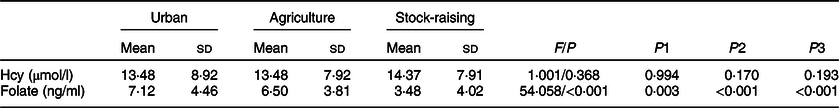
Hcy, homocysteine.
* Adjusted for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, education and ethnicity. F is the statistic value of covariance analysis; P represents the P value for among-group comparison; P1, urban v. agriculture; P2, agriculture v. stock-raising; P3, stock-raising v. urban.
Prevalence of hyperhomocysteinaemia and folate deficiency
As given in online Supplemental Table S1, the prevalence of HHcy in the stock-raising region was significantly higher than in agricultural and urban regions (82·2 v. 69·7 and 68·4 %, P < 0·001). The prevalence of HHcy in female, Kazakh, non-drinking, non-smoking, overweight and obese subjects and those with lower education attainment from the stock-raising region was significantly higher than those of other regions (P for all <0·05).
The prevalence of folate deficiency in subjects from the stock-raising region was significantly higher than in those from the agricultural and urban regions (54·8 v. 8·3 and 2·6 %, P < 0·001), which was also the same in the subgroup analysis (P for all <0·001) as shown in online Supplemental Table S2.
Relationship between homocysteine and folate
The partial correlation analysis showed a significant inverse relationship between Hcy and folate concentration (r = –0·200, P < 0·001) after controlling for age, gender, alcohol and cigarette consumption, hypertension and region as shown in Fig. 1.
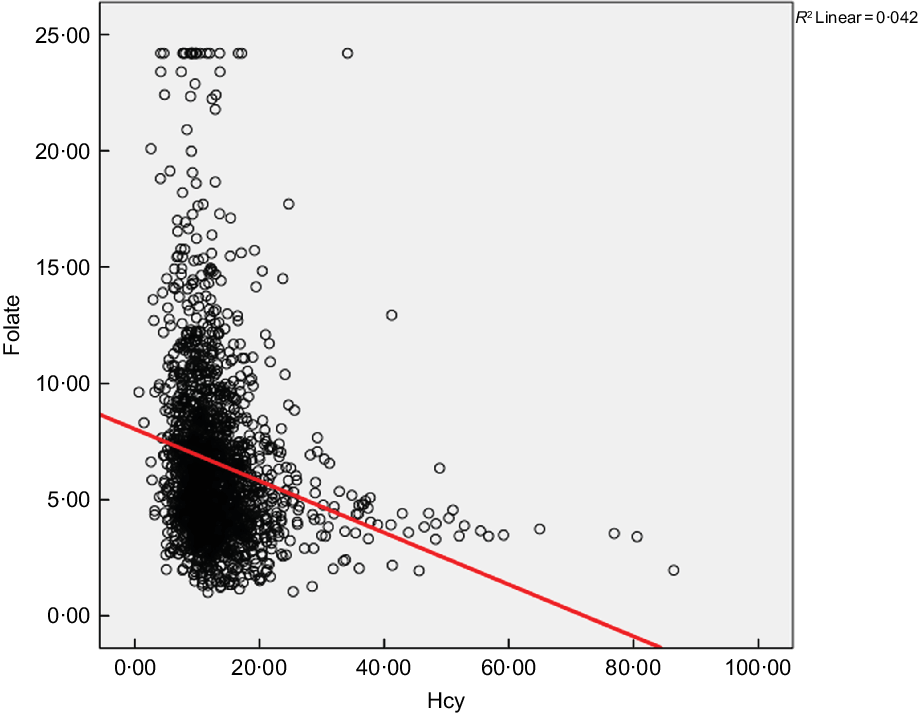
Fig. 1 Relationship between circulating homocysteine and folate in the study population, adjusted for age, gender, cigarette consumption, alcohol intake and BMI
Factors associated with hyperhomocysteinaemia and folate deficiency
Univariable and multivariate logistic regression analyses of risk factors for HHcy and folate deficiency are given in Table 3. A multivariate logistic regression analysis of risk factors for HHcy was conducted after adjusting for gender, age, BMI, alcohol and cigarette consumption, hypertension, folate tertile and regions. The OR for HHcy in the stock-raising region was 1·90 (95 % CI 1·11, 3·27) compared with the urban region after adjusting for all possible covariates. A multivariate logistic regression analysis of risk factors for folate deficiency was conducted after adjusting for gender, age, region, ethnicity and education. The OR for folate deficiency in the stock-raising and agriculture regions were 11·51 (95 % CI 7·09, 18·67) and 1·91 (95 % CI 1·30, 2·82), respectively, compared with the urban region after adjusting for all possible covariates.
Table 3 Univariable and multivariate logistic regression analyses of risk factors for HHcy and folate deficiency*
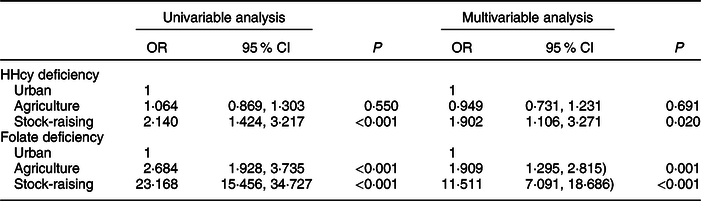
* Hyperhomocysteinaemia (HHcy) deficiency was adjusted for gender, age, BMI, alcohol intake, cigarette consumption, hypertension, folate tertile and region. Folate deficiency was adjusted for gender, age, region, ethnicity and education.
Discussion
CVD is the leading cause of human death(Reference Zhou, Wang and Zhu1), for which folate deficiency and elevated Hcy are independent risk factors(Reference Veeranna, Zalawadiya and Niraj2,Reference Wang, Li and Qin3,Reference Ebara8) . Hcy is also a sensitive marker of folate deficiency(Reference Veeranna, Zalawadiya and Niraj2,Reference Lonn, Yusuf and Arnold29) . Folate and Hcy levels vary widely due to geographical, racial/ethnic, lifestyle and dietary diversity. Xinjiang in northwest China provides an ideal setting for a study on the effects of diverse lifestyles and dietary habits across populations from urban, agricultural and stock-raising regions. The main observations of the current study encompassed(Reference Zhou, Wang and Zhu1): levels of circulating folate were the lowest in the stock-raising region(Reference Veeranna, Zalawadiya and Niraj2); the prevalence of HHcy and folate deficiency was higher in the stock-raising region(Reference Wang, Li and Qin3); stock-raising and agricultural regions were the major related factors for elevated Hcy and folate deficiency.
The current study extended previous findings to stock-raising regions(Reference Guo, Pang and Guo23). That is, the stock-raising region showed a significantly higher HHcy prevalence and higher OR for the presence of HHcy and folate deficiency. Indeed, it may be indicative of the dietary habits of the population living in the stock-raising region. As evidenced previously, stock-raisers reside in villages, forests and mountains and move around based on seasonal changes, which makes them dependent on animal products with limited access to enough vegetables and fruits(Reference Zhou, Zhao and Heizhati21). Therefore, populations from stock-raising regions may benefit most from Hcy lowering possibly by folate supplementation.
The current study also provides a direct evidence on the coexistence of HHcy and folate deficiency in populations with different lifestyles, especially in stock-raisers, from developing countries. Folate fortification is not mandatory in China, making the results possibly representative of this type of study. Consistent with previous studies(Reference Lonn, Yusuf and Arnold29), coexistence of elevated Hcy and folate deficiency and a significant inverse relationship between the two in a population from the same background may further confirm the importance of folate in terms of lowering elevated Hcy. Hcy is an intermediate product in the metabolism of methionine and cysteine. The circulation of methionine requires the participation of folate, a vitamin that human body cannot synthesise. Methylenetetrahydrofolate reductase catalyses the irreversible conversion of 5, 10-methylene tetrahydrofolate to 5-methyl-tetrahydrofolate, committing one-carbon units to the methionine cycle. When folate and/or B vitamins are deficient, methionine cycle is blocked, generating the accumulation of plasma Hcy in the blood. HHcy further induces oxidative stress, endothelium dysfunction, inflammation, smooth muscle cell proliferation and endoplasmic reticulum stress, which are the key pathogenesis of CVD(Reference Moretti and Caruso30). At present, most scholars are optimistic for the trend of folate intervention on the risk of HHcy and CVD reduction(Reference Wang, Qin and Demirtas31). With folate fortification implemented in the United States and Canada since 1998, circulating folate concentrations in the population increased from 4·6 to 10·0 ng/ml, and Hcy decreased from 10·1 to 9·4 μmol/l(Reference Qin and Huo32). Folate supplementation reduces the risk of stroke by about 10 %, and the risk of CVD by about 4 %, which is more effective in populations with lower baseline folate and at a high risk(Reference Li, Huang and Zheng33,Reference Huo, Li and Qin34) . Consistent with previous studies(Reference Qin, Wang and Qin35–Reference González-Gross, Benser and Breidenassel37), the presence of HHcy is also associated with male gender and older age. In contrast, current alcohol intake is not associated with elevated Hcy in our data, which may be due to some bias in population selection. Therefore, folate fortification, cigarette cessation and alcohol abstinence may be still the target lifestyle modification measures to lower elevated Hcy(Reference Yang, Fan and Zhi20,Reference Guo, Pang and Guo23,Reference Zhang, Liu and Zhang36) .
Moreover, this observation could be extended to settings of populations with approximate lifestyles and dietary habits. Xinjiang, located in northwest China, is close to Central Asian and Eastern European countries such as Kazakhstan, Kyrgyzstan, Mongolia and Russia. Most of the population there still share similar lifestyles (stock-raising) and dietary habits (more animal products and limited fruit and vegetables)(Reference Stefler and Bobak38,Reference Goryakin, Rocco and Suhrcke39) , where the burden of CVD seems to be huge. For instance, CVD is estimated to account for more than a half (53 %) of all deaths in Kazakhstan. Age-standardised CVD mortality was 636 in Kazakhstan and 531 in the Russia Federation per 100 000 population in 2010, almost 5–6 times higher than in the United Kingdom (112 per 100 000)(40).
The current study has following innovations: First, it extended previous findings to stock-raising regions. The prevalence of HHcy and folate deficiency was higher in stock-raisers and so they are likely the target population for folate supplementation, which may be of valuable reference to other stock-raising regions. Second, the current data included both Hcy and folate analyses, which may provide a direct evidence for disease prevention. Nevertheless, some limitations to the current study need to be acknowledged. This is a cross-sectional study that did not show a causal relationship between Hcy and folate, whereas it has been well established. Thus, our data provide a direction for the prevention and control of public health-related diseases. In addition, we failed to assess the contribution of vitamin B12 on Hcy, which may have brought some bias to our results and explanations.
In conclusion, the prevalence of HHcy and folate deficiency is unacceptably high in underdeveloped regions, particularly among stock-raisers and thus a possible target for folate supplementation. The results may be of important reference for the prevention and control of HHcy in Xinjiang, with a possibility of extension to populations that share lifestyles and dietary habits.
Acknowledgements
Acknowledgements: We thank all study participants for their support and thank volunteers for providing great help. We also thank the leaders of health bureaus of Emin county. We express our gratefulness to the Department of Science and Technology for funding this work. Financial support: The current study was funded by Department of Science and Technology of Xinjiang Uygur Autonomous Region of China (grant number 2017B03015). Conflict of interest: All the authors declared no conflict of interest. Authorship: N.L. put forward, designed and implemented the investigation, analysed the data and drafted the manuscript. F.P., M.H., L.W., L.Z, J.H., D.Z., G.C., Q.L., L.S. and N.Y. participated in investigation design and implementation, data analysis and manuscript writing. All authors have read and approved the final manuscript. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving study participants were approved by the ethics committee at People’s Hospital of Xinjiang Uygur Autonomous Region China. Written informed consent was obtained from all participants.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019004841







