1. INTRODUCTION
Marine dissolved inorganic carbon (DIC) is the largest exchangeable reservoir of carbon in the surface ocean, and it exchanges with atmospheric CO2 on annual to decadal timescales (Broecker and Peng Reference Broecker and Peng1982). As humans have released additional CO2 into the atmosphere, DIC has acted as a major sink for some of this additional carbon (Sabine et al. Reference Sabine, Feely, Gruber, Key, Lee, Bullister, Wanninkhof, Wong, Wallace and Tilbrook2004; Gruber et al. Reference Gruber, Clement, Carter, Feely, van Heuven, Hoppema, Ishii, Key, Kozyr and Lauvset2019). This increase in DIC lowers the ocean’s pH, which has profound impacts on ocean ecosystems (Feely et al. Reference Feely, Sabine, Lee, Berelson, Kleypas, Fabry and Millero2004). A key tool used to track DIC are its carbon isotopes (13C and 14C), as they are reflective of the circulation and biogeochemistry of the water mass in which DIC is dissolved. These isotopes also reflect the anthropogenic impact on the global carbon cycle. First, above-ground thermonuclear bomb testing nearly doubled the 14C content of atmospheric CO2 by the early 1960s. Due to the relatively long isotopic equilibration time of CO2, this resulted in a smaller, but longer-lived increase in surface marine DIC Δ14C (Druffel Reference Druffel1989). Second, the burning of fossil fuels since 1890 released CO2 with no 14C and less 13C than atmospheric CO2 that has reduced the overall isotopic ratios, contributing to the Suess effect (Suess Reference Suess and Aldrich1953). The decrease in 14C/12C and 13C/12C ratios have also been observed in marine DIC (Andrews et al. Reference Andrews, Siciliano, Potts, DeMartini and Covarrubias2016; Brooks Reference Brooks2020). Thus, attempts by the international community to curb CO2 emissions will likely be reflected in future DIC isotopic records.
The 13C/12C ratio of DIC is the result of equilibrium isotope effect and kinetic isotope effect acting on the sources and sinks of DIC. For example, the 13C/12C of surface DIC (δ13C ≈ 1‰) is higher than that of atmospheric CO2 (δ13C ≈ –8‰), because equilibrium processes favor a higher proportion of the heavier isotope in the more condensed phase (Mook Reference Mook1986). A non-equilibrium process, like biological uptake, favors the lighter isotope due to its higher diffusivity. Marine phytoplankton are thus, depleted in 13C (δ13C ≈ –21‰) relative to surface DIC (Mook Reference Mook1986). In regions with significant phytoplankton growth, this can also measurably raise the 13C/12C ratio of the remaining DIC (Kroopnik Reference Kroopnick1985). Conversely, remineralization of sinking marine phytoplankton by zooplankton and microbes releases 13C-depleted DIC, which then lowers the overall 13C/12C of the subsurface DIC (Kroopnik Reference Kroopnick1985).
While 14C also undergoes isotopic fractionation, reported 14C/12C ratios are corrected for any fractionation (Stuiver and Polach Reference Stuiver and Polach1977). Therefore, the primary control on a measured 14C/12C ratio is the radioactive decay of 14C and the mixing of carbon pools with different 14C/12C ratios. In the surface ocean, newly dissolved CO2 mixes with DIC from the surface water. In the pre-bomb era, this resulted in surface DIC with 14C/12C ratios (Δ14C ≈ -60) lower than that of atmospheric CO2 (Δ14C ≈ 0) (Stuiver et al. Reference Stuiver, Pearson and Braziunas1986). The equilibration time of 14C by air-sea exchange of CO2 is about 10 years, which is far longer than the mixing time of surface waters (Broecker and Peng Reference Broecker and Peng1982). Thus, shifts in the 14C/12C ratio in surface DIC during this period are almost entirely due to mixing of surface and upwelled waters within a region. The introduction of bomb radiocarbon to the atmosphere produced a large isotopic gradient with the ocean that increased the Δ14C of surface DIC by ∼200‰ (Druffel et al. Reference Druffel, Beaupré, Ziolkowski, Schuur, Druffel and Trumbore2016). As the 14C/12C of atmospheric CO2 has declined due to redistribution of bomb-derived 14C and the 14C-free CO2 from fossil fuels, the surface DIC 14C/12C ratios have also declined, albeit at a slower rate (Hinger et al. Reference Hinger, Santos, Druffel and Griffin2010; Santos et al. Reference Santos, Ferguson, Acaylar, Johnson, Griffin and Druffel2011; Andrews et al. Reference Andrews, Siciliano, Potts, DeMartini and Covarrubias2016). In the last few years, atmospheric CO2 reached its pre-bomb 14C/12C ratio (Δ14C≈0‰) (Graven et al. Reference Graven, Keeling and Xu2022). How this ratio and the resulting change in surface DIC carbon isotope ratios will change in the coming years will require continuous monitoring of these carbon pools.
One such monitoring site is located at Newport Beach, California in the Southern California Bight (SCB). The SCB is home to productive marine ecosystems and is characterized by complex circulation of local currents. The northern end of the SCB is at Point Conception (∼34.4°N), where the North American coastline turns almost 90° westward and then begins curving southwards (Figure 1). The SCB ends 236 km south of the Mexican-American border, in Baja California (∼32°N). The eastern boundary current of the North Pacific Gyre, the California Current, flows southward from Point Conception and dominates the western portion of the SCB (Hickey Reference Hickey1979). This current begins in the subarctic region west of Washington state and is relatively low in temperature and salinity. Closer to the coast, there is the poleward flowing Southern California Countercurrent that brings warmer, nutrient-depleted waters from Baja California. These two currents create a domain scale gyre that can be subdivided into 3 cyclonic eddies (Dong et al. Reference Dong, Idica and McWilliams2009). These eddies can transport warmer, nutrient-poor water east from the North Pacific Gyre to the SCB (Dong et al. Reference Dong, Idica and McWilliams2009). The Southern California Countercurrent dissipates in the spring when wind-driven upwelling creates a westward flow that brings colder, nutrient-rich waters to the surface and stimulates phytoplankton growth (Bray et al. Reference Bray, Keyes and Morawitz1999). However, the upwelling in this region is generally weaker than at other points along the Eastern North Pacific (Hickey Reference Hickey1992). The proportions these different water masses present in the SCB at a given time vary based on the prevailing winds (Hickey et al. Reference Hickey, Dobbins and Allen2003).
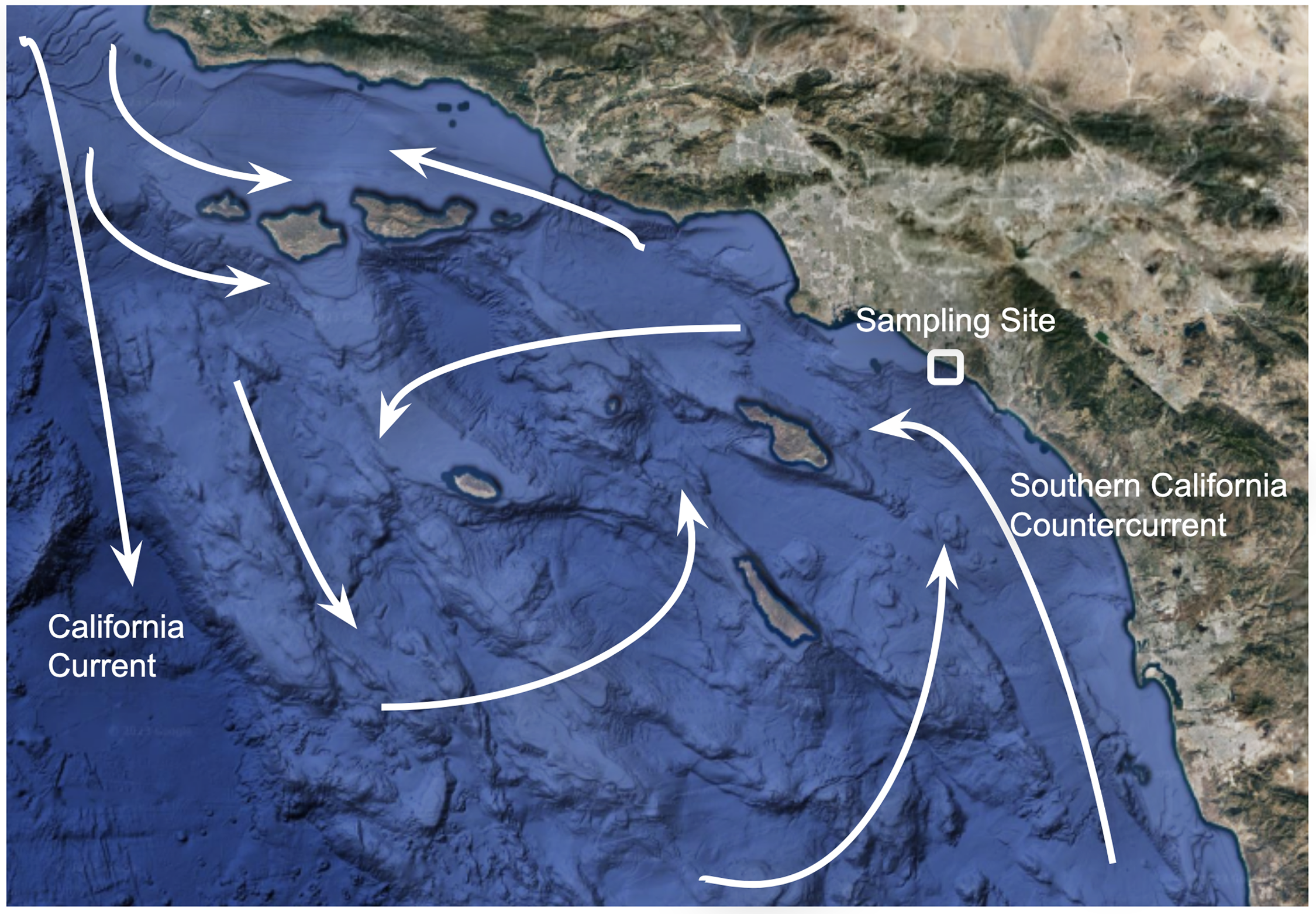
Figure 1 Google Earth image of the Southern California Bight. Arrows show surface currents as described in Hickey (Reference Hickey1992). Google, Data LDEO-Columbia, NSF, NOAA, Landsat/Copernicus Data SIO, NOAA, U.S. Navy, NGA, Gebco Data MBARI.
The main subsurface flow in the SCB is the California Undercurrent. This current is formed in the Eastern Equatorial Pacific and is characterized by warm, salty, nutrient-replete, and low-oxygen water (Hickey Reference Hickey1979). Its core generally resides from 200-300 m depth and rises to 100 m during the spring upwelling (Dong et al. Reference Dong, Idica and McWilliams2009; Brogard et al. Reference Bograd, Schroeder and Jacox2019). CFC-ages between 200–300 m in the SCB indicate that the California Undercurrent’s ventilation age is around 50–125 years (Jeanson et al. Reference Jeansson, Steinfeldt and Tanhua2021; Figure S1). DIC Δ14C values from this depth in the SCB have been observed to be 40-90‰ lower than surface DIC values (Figures S2a and S2b) (Key et al. Reference Key, Olsen, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2015; Olsen et al. Reference Olsen, Key, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2016). This offset cannot be translated directly into a radiocarbon age due to the presence of bomb radiocarbon. Upwelling results in surface DIC in the SCB with Δ14C values that are 50‰ lower than those of surface DIC from the North Pacific Gyre (Figures S3a and S3b) (Key et al. Reference Key, Olsen, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2015; Olsen et al. Reference Olsen, Key, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2016). As expected, δ13C values of DIC are lower within the California Undercurrent than those in the surface waters of the SCB (Figure S2c) (Key et al. Reference Key, Olsen, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2015; Olsen et al. Reference Olsen, Key, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2016). However, the surface DIC δ13C values in the SCB are higher than those in the North Pacific Gyre, suggesting that upwelling is not the primary driver of δ13C values in the SCB (Figure S3c) (Key et al. Reference Key, Olsen, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2015; Olsen et al. Reference Olsen, Key, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2016).
During the past decade, the SCB has had unusually warm temperatures and low rainfall (Frankson et al. Reference Frankson, Stevens, Kunkel, Champion, Easterling, Sweet and Anderson2022). In 2012, the region entered a drought, due to the formation of a persistent ridge of high atmospheric pressure over the Northeast Pacific (Seager et al. Reference Seager, Hoerling, Schubert, Wang, Lyon, Kumar, Nakamura and Henderson2015). Sea surface temperatures (SST) also abruptly rose due to ocean heat waves between 2013–2015 and 2018–2020 (Bond et al. Reference Bond, Cronin, Freeland and Mantua2015; Weber et al. Reference Weber, Auth, Baumann-Pickering, Baumgartner, Bjorkstedt, Bograd, Burke, Cadena-Ramírez, Daly and de la Cruz2021). Due to anthropogenic climate change, the region is expected to continue to warm and to oscillate between extreme dry and extreme wet periods (Berg and Hall Reference Berg and Hall2015). The distinct water masses that contribute to the SCB and the region’s sensitivity to climate change make it valuable for understanding both the natural variability of DIC carbon isotopes and the human impact on them.
Hinger et al. (Reference Hinger, Santos, Druffel and Griffin2010) and Santos et al. (Reference Santos, Ferguson, Acaylar, Johnson, Griffin and Druffel2011) reported DIC Δ14C and δ13C measurements of seawater from the Newport Beach Pier in the Southern California Bight between 2004 and 2010. Here, we present a continuation of this time series that contains mostly monthly records from the Newport Beach Pier between 2011 and 2022. Combined, the nearly two decades of data reflect the natural variability of DIC, the response of DIC to a changing climate, and the effect of fossil fuel CO2 emissions on the carbon isotopes in DIC.
2. METHODS
2.1 Sample Collection
Sampling was performed monthly at the Newport Beach Pier in Orange County, California (33°36'21"N, 117°55'52"W). Sampling has been conducted since August 2004, with varying gaps in 2009, 2016, 2018, and 2020.
Glass bottles (0.25-L media bottles) were acidified in 10% HCl for 2 hr, rinsed with Milli-Q water, baked at 540°C for 2 hr, and then stored in plastic bags. Sea water was collected via a surface cast of a plastic bucket on a nylon line. The bucket was fitted with a spigot and Teflon tubing that was used to fill the sample bottles. Sample bottles were rinsed three times with sea water and then filled and overflowed for one volume. Samples were poisoned with two drops of saturated mercuric chloride solution and inverted several times after closure. Duplicates were collected for each sampling.
2.2 DIC Extraction
The DIC extraction for 14C samples was performed according to the procedure described by Gao et al. (Reference Gao, Xu, Zhou, Pack, Griffin, Santos, Southon and Liu2014). Briefly, ∼45 mL of seawater was subsampled into a 60-mL vial fitted with a Teflon and a Viton septa inside a He-filled glove box. The samples were then acidified with 0.5 mL 85% H3PO4 administered using a Hamilton glass syringe with a Sub-Q 26G5/8 gauge needle. The sample vials were then heated on a heatblock at 75°C for 2 hr to convert all DIC to gaseous CO2. The CO2 from the headspace of the sample vial was extracted by a 60-mL syringe with a one-way stopcock. The CO2 was then loaded onto a vacuum line through a septum for cryogenic purification. Samples were converted to graphite using the sealed tube zinc reduction method over iron catalyst as described by Xu et al. (Reference Xu, Trumbore, Zheng, Southon, McDuffee, Luttgen and Liu2007).
The DIC extraction for 13C samples was performed according to a modified procedure described by Torres et al. (Reference Torres, Mix and Rugh2005). One mL of seawater was subsampled into a Labco exetainer vial fitted with a Labco septa in a He gas-filled glove box. The samples were then acidified with 50 µL of 85% H3PO4 administered with a BD Falcon 1 mL syringe with a 26G5/8 gauge needle. Samples were allowed to equilibrate at room temperature for 12 hr to prevent 13C fractionation of the CO2.
2.3 Isotope Analyses
The 14C analyses of the graphite samples were performed at the Keck Carbon Cycle Accelerator Mass Spectrometry (KCCAMS) Laboratory at the University of California, Irvine (Beverly et al. Reference Beverly, Beaumont, Tauz, Ormsby, von Reden, Santos and Southon2010). Process standards and blanks were performed by dissolving modern coral standard (CSTD), IAEA-C2 and radiocarbon-dead calcite in separate aliquots of previously acidified and stripped sea water using the 13C DIC extraction described above. The results from the calcites were used to correct for sample preparation backgrounds added during DIC extraction and graphitization of samples (Gao et al. Reference Gao, Xu, Zhou, Pack, Griffin, Santos, Southon and Liu2014). Radiocarbon results are reported as Δ14C corrected for collection date (Stuiver and Polach Reference Stuiver and Polach1977). Uncertainty of Δ14C was estimated as ±2.6‰ (±1σ, 31 data points) using the pooled, standard deviation of replicate measurements (McNaught and Wilkinson Reference McNaught and Wilkinson1997).
The 13C analysis was performed at UCI using a Gas Bench II coupled with a Thermo Scientific Delta Plus XL isotope ratio mass spectrometer. CO2 from the sample preparation vials was directly transferred into the Gas Bench via an auto-sampler. DIC concentration was determined with the same IRMS, using a calibration curve derived from calcite standards with various weights. The pooled standard deviation of repeat measurements of δ13C was estimated as ±0.05‰ (±1σ, 14 data points). However, due to uncertainties in the δ13C values of the standards, we report an uncertainty of 0.1‰ for δ13C values. Uncertainty of the concentrations was ±0.01 mM C based on the pooled standard deviation of repeat measurements (±1σ, 14 data points).
2.4 Sea Surface Characteristics
Sea surface temperature (SST) and salinity data were obtained from the Scripps Shore Stations program (Carter et al. Reference Carter, Flick, Terrill, Beckhaus, Martin, Fey, Walker, Largier and McGowan2022). Newport Beach lifeguards collected temperatures and water samples daily and sent the samples to Scripps for analysis. The water samples were analyzed for salinity at Scripps Institution of Oceanography (https://sccoos.org/autoss). The Coastal Upwelling Transport Index (CUTI), from NOAA Pacific Fisheries Environmental Laboratory, was used to determine wind-driven upwelling rates at the site (https://oceanview.pfeg.noaa.gov/products/upwelling). CUTI uses satellite and in situ wind measurements to estimate the vertical transport of water (Jacox et al. Reference Jacox, Edwards, Hazen and Bograd2018). Our site is very close to the boundary between the 33°N and 34°N CUTI grid boxes. We selected the 33°N box to remain consistent with prior studies (Santos et al. Reference Santos, Ferguson, Acaylar, Johnson, Griffin and Druffel2011).
3. RESULTS
3.1 DIC Concentration and Isotopes
The DIC Δ14C, δ13C values and DIC concentrations are shown in Table 1 (see Appendix) and Figures 2a–c. The DIC Δ14C values ranged from 22.1‰ in March to –12.6‰ in December 2021 (Figure 2a). The DIC δ13C values ranged from 2.14‰ in April 2014 to –0.30‰ in October 2015 (Figure 2b). Concentrations varied from 2.04 mM C in October 2020 to 2.68 mM C in August 2013 and the average concentration was 2.29 mM C (n = 89) (Figure 2c). The samples collected did not coincide with any major precipitation or runoff events.

Figure 2 Time series of (a) Δ14C values, (b) δ13C values, and (c) concentration of surface DIC, (d) the 15-day moving average of CUTI, (e) sea surface temperature, and (f) surface salinity of water samples. Error bars from this work represent the pooled standard deviations of repeated analyses of samples. Error bars for DIC concentration are smaller than the sizes of the symbols. Dashed lines indicate the different sources of data.
3.2 Oceanographic Conditions in the Surface Waters
The 15-day moving average of CUTI ranged from 1.5 m2/s to –0.2 m2/s (Figure 2d). Upwelling was typically elevated in the spring and summer months and lower during the winter months. SST ranged from 10.0°C in the winter to 25.2°C in the summer (Figure 2e). Lows in SST during the summers of 2010 and 2017 are likely due to enhanced upwelling or intrusion of the colder California Current water towards the coast. SST increased markedly in 2014 and remained elevated throughout 2022. Surface salinities ranged from 23.39 practical salinity units (PSU) to 34.87 PSU throughout the 2011–2022 period (Figure 2f). Salinity was lowest and most variable during the winter months and highest during the summer and early fall.
4. DISCUSSION
We present the discussion in three parts. First, we discuss the possible reasons for the decline of the DIC Δ14C and δ13C values over the past two decades. Second, we discuss the seasonal trends in the DIC Δ14C and δ13C values and the driving factors behind these trends. Third, we discuss how major climate events between 2011–2022 may have affected the DIC Δ14C and δ13C values and their trends.
4.1 Decline of DIC Δ14C and δ13C Values
The long-term trends in Δ14C and δ13C values were evaluated by performing model 2 geometric regressions of the average annual isotopic values versus the number of years since measurements began in 2004. The regressions were performed using the python package Scipy (Virtanen et al. Reference Virtanen, Gommers, Oliphant, Haberland, Reddy, Cournapeau, Burovski, Peterson, Weckesser and Bright2020). These analyses included years with sampling gaps because omission of these years did not change the slope coefficients of the trendlines by more than one standard error.
Annual average DIC Δ14C values decreased linearly from 35‰ in 2004 to –6‰ in 2022 (R2=0.96, p<<0.001) (Figure 3a). This is consistent with atmospheric CO2 observations from La Jolla, California, (32.9ºN, 117.3ºW), also in the SCB, where atmospheric CO2 Δ14C decreased from 60‰ to –5‰ over this same time period (Graven et al. Reference Graven, Keeling and Xu2022). This indicates that air-sea CO2 exchange is a significant factor in the long-term trend of surface DIC Δ14C. Prior to anthropogenic influence, surface DIC Δ14C values were lower than those of atmospheric CO2 due to mixing of surface water with aged water masses and the slow equilibration time (∼10 years) of atmospheric and dissolved 14CO2 (Broecker and Peng Reference Broecker and Peng1982). This mixing with aged subsurface waters means that the surface DIC Δ14C has a lagged and dampened response to the anthropogenic disturbances in atmospheric CO2 Δ14C. In recent years, these two reservoirs have converged. In 2000, annual coral bands in the North Pacific Gyre had Δ14C values that were higher than atmospheric CO2 values (86‰) (Andrews et al. Reference Andrews, Siciliano, Potts, DeMartini and Covarrubias2016), and higher surface water DIC Δ14C than maritime air in 2014 from the South China Sea was reported by Gao et al. (Reference Gao, Zhou, Liu and Xu2018). Our coastal site has lower DIC Δ14C values than surface gyre water due to the local upwelling. As of 2020, DIC Δ14C values at Newport Beach pier and atmospheric 14CO2 values are both below 0‰ and within experimental error of one another (Graven et al. Reference Graven, Keeling and Xu2022). The future relationship of DIC and CO2 Δ14C values at this site is highly dependent on the magnitude of fossil fuel CO2 emissions in the coming years. Thus, continued monitoring of this site will be invaluable for understanding the magnitude of the ocean carbon sink and the efficacy of our efforts to mitigate climate change.

Figure 3 Annual average of (a) Δ14C and (b) δ13C values of DIC samples from Newport Beach Pier. Dashed lines show model 2 geometric regressions. Error bars represent standard deviation of analyzed samples from that year.
Annual average DIC δ13C values decreased from 1.6‰ in 2004 to 1.2‰ in 2022 (R2 = 0.50, p = 0.001) (Figure 3b). This trend is consistent with decreasing δ13C of atmospheric CO2 (Quay et al. Reference Quay, Sonnerup, Munro and Sweeney2017) and in the North Pacific Gyre (Brooks Reference Brooks2020). This is likely a further demonstration of the δ13C Suess effect as fossil fuel CO2 emissions continue. It should be noted that the annual variability of δ13C (0.7‰) is greater than the total decline observed during this 18-year period (0.4‰). This suggests the possibility that strong seasonality or significant changes in local carbon cycling could mask the Suess effect on DIC δ13C in small data sets.
4.2 Seasonality of DIC Δ14C and δ13C
Seasonality was evaluated by comparing the average monthly Δ14C and δ13C values for the entire data set. As the change in Δ14C from 2004–2022 was found to be larger than the annual variation during this time, the Δ14C values were detrended assuming a linear trend using Scipy (Virtanen et al. Reference Virtanen, Gommers, Oliphant, Haberland, Reddy, Cournapeau, Burovski, Peterson, Weckesser and Bright2020). The annual variation in δ13C values was larger than the change in annual averages from 2004 to 2022, so detrending was not performed. Each year was given equal weight when determining monthly averages to account for some years with multiple samples in a single month (Figure 4). Samples on days with salinity <32 PSU were omitted from this analysis. This was done to remove the effect of precipitation events, because changes from these events are highly variable and short-lived (Hinger et al. Reference Hinger, Santos, Druffel and Griffin2010). These analyses were performed separately for data from Hinger et al. (Reference Hinger, Santos, Druffel and Griffin2010) and Santos et al. (Reference Santos, Ferguson, Acaylar, Johnson, Griffin and Druffel2011) (Figures 3a and 3c) and for the data from this study (Figures 3b and 3d).

Figure 4 Monthly average of (a) and (b) Δ14C and (c) and (d) δ13C values of DIC samples from Newport Beach Pier from the (a) and (c) prior timeseries and this work (b) and (d). Error bars represent standard error of the mean for samples available from each month. Samples from 2004–2010 are from Hinger et al. (Reference Hinger, Santos, Druffel and Griffin2010) and Santos et al. (Reference Santos, Ferguson, Acaylar, Johnson, Griffin and Druffel2011). Samples from 2011–2022 are from this work.
Between 2004–2010 (Figure 3a), detrended Δ14C values are elevated throughout the winter and early spring, followed by a sharp drop in May and gradual rise in the summer and autumn. Hinger et al. (Reference Hinger, Santos, Druffel and Griffin2010) attributed the elevated Δ14C values in the winter to an increase in the number and clustering of small eddies during the winter months. They hypothesized that these eddies transported North Pacific gyre water with higher Δ14C values to our site. After 2011, the elevated winter values are not present (Figure 3b). We hypothesize that this could have occurred for two reasons. First, there was less transport of gyre water to our site. During our study period, an atmospheric ridge of persistent high-pressure was formed over the Northeast Pacific (Seager et al. Reference Seager, Hoerling, Schubert, Wang, Lyon, Kumar, Nakamura and Henderson2015). This ridge drastically reduced the magnitude of the winter winds that possibly could have reduced the eddy strength during this time (Seager et al. Reference Seager, Hoerling, Schubert, Wang, Lyon, Kumar, Nakamura and Henderson2015). Second, the DIC Δ14C values of the Gyre waters have also decreased during this period (Figure S3) (Key et al. Reference Key, Olsen, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2015; Olsen et al. Reference Olsen, Key, van Heuven, Lauvset, Velo, Lin, Schirnick, Kozyr, Tanhua and Hoppema2016). As the Δ14C values of the surface and upwelled waters converge, this may reduce the strength of the seasonal cycle.
The detrended DIC Δ14C values from this study period (Figure 3b) display semiannual seasonality with small peaks in March and November and troughs in January and September. The DIC Δ14C values vary by 3.4‰ during these cycles, which is less than 2 times the largest standard error in March (2.1‰). The lower DIC Δ14C values in winter and summer are likely indicative of mixing between surface water and deeper, older water masses. Upwelling is at its maximum during the late spring, and we observe a decrease in DIC Δ14C values during this time. Upwelling is stronger further North in the California Current, and transport of these upwelled waters to the SCB may have continued to keep the Δ14C values lower during the months after the upwelling maximum (Hickey Reference Hickey1992). The low Δ14C values during the winter, when upwelling is weak, could be due to deepening of the mixed layer depth that allows for advection of the deeper, aged water to the surface. The peaks in spring and autumn likely then reflect an increase in the contribution of water from the North Pacific Gyre, which have higher Δ14C values (Andrews et al. Reference Andrews, Siciliano, Potts, DeMartini and Covarrubias2016). This trend overall suggests a steady composition of source waters at our site.
Average seasonal δ13C values are strongly seasonal with a minimum during winter and a maximum during summer (Figures 3c and 3d). The seasonality of the two study periods is largely the same, except that, on average, the δ13C values were 0.2‰ lower during 2011–2022 than in 2004–2010. The summer maximum suggests that mixing of surface and upwelled water is not the primary control of δ13C. Deep water DIC typically has lowered δ13C due to the remineralization of particulate and dissolved organic matter (Kroopnick Reference Kroopnick1985). If mixing with upwelled water was the main driver of DIC δ13C variability, then we would expect to see decreases in δ13C values during the summer months, as we do with Δ14C values. Instead, we find a pattern similar to atmospheric δ13C CO2 values. Atmospheric fluctuations in δ13C CO2 values are due to fractionation during terrestrial photosynthesis, which preferentially removes 12C atoms. We hypothesize that the surface DIC δ13C at our site fluctuates due to a similar mechanism. As nutrient-rich waters from spring and summer upwelling stimulate primary productivity, the phytoplankton and kelp take up DIC with lower δ13C values and thereby increase the δ13C of DIC remaining in the water.
The average increase in δ13C between March and August is 0.7‰ (Figure 3d). Assuming a concentration of DIC in March of 2.25 mM C (Figure 2c), a DIC δ13C of 1.0‰, and a δ13C of phytoplankton of –21.0‰, we calculated that 3% (0.07 mM C) of the DIC would need to be fixed by the phytoplankton to produce this increase. Particulate organic carbon concentrations at this site vary seasonally by about 0.03 mM C (Fagan et al. Reference Fagan, Moreno and Martiny2019). Assuming a dissolved organic carbon production of a similar magnitude, which is typical in marine settings (Carlson et al. Reference Carlson, Ducklow, Hansell and Smith1998), this suggests that there is sufficient biological fractionation to account for the seasonal variation of DIC δ13C. This seasonality may be a coastal phenomenon because coasts typically have much higher phytoplankton concentrations than the rest of the ocean (Antione et al. Reference Antoine, André and Morel1996).
4.3 Major Upwelling Event Reflected in DIC Δ14C and δ13C
During the late winter and spring of 2016, we observe an increase in DIC concentration and salinity and decreases in the Δ14C and SST (Figures 2a, 2c, 2e, 2f). These features are all consistent with a period of strong upwelling. The CUTI index does show strong fall and winter upwelling during this time period (Figure 2d). This has been attributed to the abrupt end of the 2015–2016 El Niño that resulted in strong upwelling winds (Frischknecht et al. Reference Frischknecht, Münnich and Gruber2017). This unseasonal upwelling created a large positive nutrient anomaly in the region that stimulated higher than normal phytoplankton growth during the winter and early spring. This reduced the amount of nutrients available during the 2016 summer and consequently reduced the phytoplankton abundance during that summer (Frischknecht et al. Reference Frischknecht, Münnich and Gruber2017). During the summer of 2016, the DIC δ13C values are lower than other summers (Figure 2b). This is consistent with the hypothesis that primary productivity is the dominant control of δ13C in this region.
5. CONCLUSION
This work provides an extended timeseries of surface DIC δ13C and Δ14C from the Newport Beach Pier for two decades. This series demonstrates the seasonality due to changes in ocean circulation and the continued dilution of these isotopes due to CO2 from fossil fuel sources. DIC δ13C values decreased by 0.03‰ per year with a total decrease of 0.4‰ from 2004 to 2022. DIC Δ14C values decreased by 2‰ per year with a total decrease of 42‰ from 2004 to 2022. Between 2004 and 2010, seasonal monthly average Δ14C values varied by 11‰ and between 2011 and 2022, monthly average Δ14C values varied by 3.4‰. The Δ14C variability was likely driven by vertical mixing bringing 14C-depleted waters to the surface and offshore eddies bringing 14C-enriched waters from the gyres to the coastline. Monthly averaged δ13C values vary by 0.7‰, likely driven by marine primary productivity, similar to atmospheric CO2. The seasonal signal in Δ14C is smaller during 2011–2022 than during 2004–2010, but the signal does still correspond to seasonal upwelling, including a major upwelling event in 2016. As δ13C and Δ14C of both atmospheric CO2 and surface DIC continue to decline, their relative values may provide vital insight to the rate and magnitude of the fossil fuel CO2 sink in the ocean, as well as climatic shifts that affect ocean circulation.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/RDC.2023.73
ACKNOWLEDGMENTS
We thank John Southon and all W.M. Keck Carbon Cycle AMS members for their assistance with the 14C and 13C samples. We thank the Newport Beach Lifeguard for the collection of daily temperature and salinity samples as part of the Scripps Shore Stations program. This work was supported by the NSF Chemical Oceanography Program (OCE–1951073) and the Fred Kavli Foundation.
APPENDIX
Table 1 Δ14C values, δ13C values, and concentrations of surface DIC samples.









