Childhood adverse experiences, including abuse and neglect, increase risk for major depressive disorder (MDD) and suicidal behaviour in adulthood.Reference Widom, DuMont and Czaja1 Childhood adversity reduces brain serotonergic (5-HT) neurotransmission implicated in MDD, aggressive behaviour and suicidal behaviour.Reference van Heeringen and Mann2 However, it is not clear how childhood adversity or recent life stressors may affect the serotonergic system via epigenetics or how epigenetic effects may increase the risk for MDD and other psychopathology in adulthood. Genetic polymorphisms of the 5-HT1A receptor gene have been associated with MDD in a meta-analysis,Reference Kishi, Yoshimura, Fukuo, Okochi, Matsunaga and Umene-Nakano3 and early-life stress may moderate this association,Reference Zhang, Xu, Xu, Yang, Luo and Sun4 potentially via epigenetic effects, as it has been associated with DNA methylation of serotonergic pathway genes.Reference Kang, Kim, Stewart, Kim, Bae and Kim5 One of the single nucleotide polymorphisms (SNPs) of the 5-HT1A receptor gene (−1019C/G; rs6295)Reference Wu and Comings6 that regulate its expressionReference Lemonde, Turecki, Bakish, Du, Hrdina and Bown7 has a G allele with lower affinity for inhibitory transcription factors.Reference Czesak, Lemonde, Peterson, Rogaeva and Albert8 MDD is associated with a higher frequency of the rs6295 G/G genotype,Reference Lemonde, Turecki, Bakish, Du, Hrdina and Bown7 and this genotype has been associated with greater 5-HT1A binding in the raphe nuclei.Reference David, Murthy, Rabiner, Munafó, Johnstone and Jacob9 Methylation of two CpG sites (−681 and −1007) increases autoreceptor expression, reducing serotonin release and affecting psychiatric illness and treatment response.Reference Le Francois, Soo, Millar, Daigle, Le Guisquet and Leman10
We hypothesised a potential mediator role for DNA methylation at the regulatory sites listed above in the upstream promoter region of this gene (−681, −1007 and −1019) for the effect of childhood and recent stress on expression in the brain, potentially moderated by diagnosis for recent stress. To our knowledge, this is the first study to examine associations between (a) 5-HT1A promoter genotype, childhood and recent life stress and (b) blood mononuclear cell DNA methylation and 5-HT1A binding potential in brain, in order to better understand the role of 5-HT1A binding as it relates to effects of stress on MDD.
Method
Participants
The sample included 192 participants with MDD (165 in a current major depressive episode) and 88 healthy volunteers (controls). MDD diagnosis was determined by DSM-IV criteria according to the Structured Clinical Interview for Axis I Disorders.Reference First MB, Gibbon and Williams11 Positron emission tomography (PET) 5-HT1A receptor binding potential (BPF) was available for 119 participants (62%), including 69 with MDD and 50 controls. Subsets of the PET imaging data have been previously utilised in papers examining 5-HT1A binding in people with depression and controls during an episode of MDD or between episodes.Reference Parsey, Olvet, Oquendo, Huang, Ogden and Mann12–Reference Miller, Brennan, Ogden, Oquendo, Sullivan and Mann15 Exclusion criteria included fluoxetine use within 6 weeks of PET scanning, or exposure to a 5-HT1A receptor agonist such as antipsychotic medications within 6 months of scanning. Participants in the MDD group on other antidepressant treatment at study enrolment underwent a medication washout and were drug-free for at least 2 weeks prior to neuroimaging, with the exception of short-acting benzodiazepines, which could be used as needed for anxiety/insomnia up to 72 h prior to scanning. The control participants had no history of DSM-IV Axis I or Axis II psychiatric disorders, no psychotropic medication exposure and no family history of a mood disorder. Exclusion criteria common to both groups included presence of significant active medical conditions, recent alcohol or other substance use disorder, dementia, neurological disease, head injury with loss of consciousness, pregnancy, first-degree family history of schizophrenia if younger than 33 years old and greater than three lifetime exposures to 3,4-methylenedioxymethamphetamine.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human participants/patients were approved by the Institutional Review Board of the New York State Psychiatric Institute, New York, NY, USA, in protocol number 6786. All participants provided informed written consent after an explanation of the study protocol and associated risks. Data on ethnicity were collected using self-report according to categories as required by the funding agency (National Institute of Mental Health).
Assessment of childhood adversity and recent life stress
Childhood physical or sexual abuse was self-reported as present or absent during a semi-structured interview in 259 participants (93%) and was coded yes/no. Recent life stressors in the previous 2 years were recorded in 220 participants (79%) using the Recent Life Changes Questionnaire (RLCQ),Reference Rahe16 which measures stressors in the previous four 6-month epochs on 76 items grouped in five domains: health; work; home/family; personal/social; and financial. Weights (in life change units, or LCU) for each item on the scale were as previously established.Reference Miller and Rahe17
Genotyping and methylation
Participants were genotyped at SNP rs6295 on the 5-HT1A receptor gene (details are given in the Supplementary material, available at https://dx.doi.org/10.1192/bjp.2023.13). The Hardy–Weinberg equilibrium test in the control group was not significant (P = 0.084). DNA methylation at the −1019 CpG site was assayed except for GG genotypes, which are not methylated, and five other participants had missing values, at the −1007 CpG site in all participants and at the −681 CpG site in 279 (99.6%) of participants (see the Supplementary material for methodological details).
PET measurement of 5-HT1A binding potential
[11C]WAY100635 was administered as a bolus over 30 s for quantification of 5-HT1A binding. Preparation of [11C]WAY100635 was as previously described.Reference Parsey, Slifstein, Hwang, Abi-Dargham, Simpson and Mawlawi18 Radiotracer radioactivity in arterial plasma, parent fraction and free fraction in plasma (f P) were determined.Reference Parsey, Hastings, Oquendo, Huang, Simpson and Arcement19 Unmetabolised parent fraction levels were fit with a Hill function and then multiplied by the total radioactivity levels in plasma. The resulting metabolite-corrected arterial data were fitted using a straight line and the sum of three decreasing exponentials as the model before and after the curve peak respectively, to generate the final metabolite-corrected arterial input function (AIF). PET images were acquired with an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, Tennessee). A T1-weighted magnetic resonance imaging (MRI) scan was acquired for each participant for co-registration with PET images. Thirteen brain regions of interest (ROIs) were chosen a priori based on areas of abundant binding: the raphe nuclei, anterior cingulate, cingulate, dorsal prefrontal cortex, hippocampus, insula, medial prefrontal cortex, parietal cortex, parahippocampal gyrus, occipital cortex, orbital cortex, temporal cortex and amygdala. Cerebellar white matter was used as a reference region.Reference Parsey, Ogden, Miller, Tin, Hesselgrave and Goldstein13 All ROIs except for the raphe nuclei were identified on each individual T1-weighted MRI using a previously described algorithm.Reference Parsey, Ogden, Miller, Tin, Hesselgrave and Goldstein13 Briefly, ROIs were drawn by trained technicians to approximate brain atlases and published reports and then refined using the segmented MRI. Owing to their small size, raphe nuclei were labelled using a standard space mask of the average location of the raphe nuclei in 52 healthy participants, created using [11C]WAY100635 voxel binding potential maps.Reference Delorenzo, Delaparte, Thapa-Chhetry, Miller, Mann and Parsey20
Binding measure estimation
Arterial plasma radioactivity, metabolites and plasma free fraction (f P) were collected and assayed as previously described.Reference Parsey, Olvet, Oquendo, Huang, Ogden and Mann12 Total volumes of distribution (V T) of [11C]WAY100635 were estimated for each ROI using the AIF and a two-tissue compartment constrained (2TCC) model. Briefly, each ROI's time activity curve was fitted with a 2TCC model in which the ratio of two of the model's free parameters (K 1/k 2, representing tracer non-displaceable volume of distribution) was constrained to equal that of the reference region. The outcome measure, binding potential BPF, was calculated as (V T(ROI) – V T(REF))/f P, where V T(ROI) is the V T in a specific ROI and V T(REF) is the V T in the reference region.
Statistical method
All statistical analyses were performed in R version 4.09 run on MacOS.21 DNA methylation values and BPF were log-transformed and outliers, defined as more than 1.5 interquartile range below the first quartile or above the third quartile, were censored to the nearest non-outlier value (less than 4% of values per variable). Genotype was coded as a three-level factor, although models with allele number were also explored. For models including methylation at the −1019 CpG site, GG genotypes were excluded, as that genotype cannot be methylated. Since childhood adversity was rare in the control group (reported by n = 5), all analyses involving childhood adversity were run only in the MDD group.
In preliminary analyses, logistic regression tested for association of childhood adversity history with genotype, age and gender. Recent stress in all participants was modelled in a multiple regression model as a function of genotype, diagnosis, age and gender.
The analysis was performed in three steps. First, associations between childhood adversity or recent stress and gene methylation level at the three sites were tested separately using linear regression, with methylation as dependent variable and stress/adversity, genotype, their interaction, age and gender as independent variables. Non-significant interactions were removed. Second, BPF was modelled as a function of stress or adversity, genotype and their interaction, with age and gender as covariates. For recent stress, diagnostic group was included both as a main effect and through interaction with stress. Third, BPF was modelled as a function of methylation, including group as main effect and in interactions. For BPF, first mixed-effects models were run including brain region as a main effect and through interaction with each predictor, and subject-specific random intercepts. Observations were weighted inversely proportionally to the squared standard errors of the BPF estimates, calculated via bootstrapping, as described elsewhere.Reference Ogden and Tarpey22 When interactions of predictors with brain region were significant, weighted least squares analyses were run at the region level. Analyses were not adjusted for multiple testing.
Results
There were no significant differences between the MDD and control groups in age or gender (Table 1). Childhood physical or sexual abuse was reported by 6% of the control group, compared with 33% of the MDD group. Recent stress was slightly more severe in the MDD group compared with controls (Cohen's d = 0.46, t = 3.28, d.f. = 218, P = 0.0012), but in the MDD group did not correlate with depression severity on the Hamilton Rating Scale for Depression (Spearman r s = 0.02, P = 0.8501) or with childhood adversity (d = 0.29, t = 1.56, d.f. = 132, P = 0.1219).
Table 1 Demographic and clinical characteristics of the sample (n = 280)
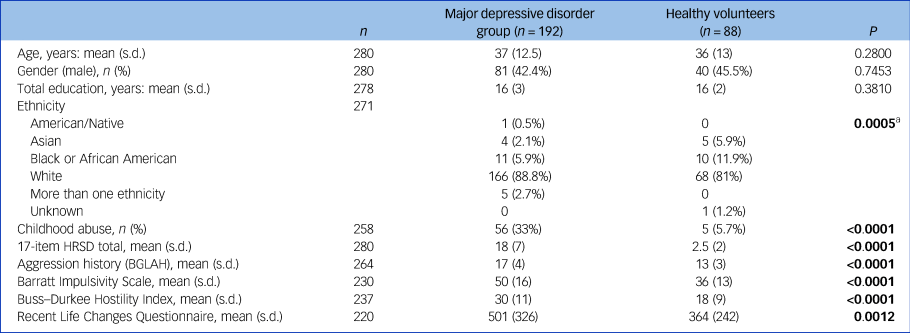
a. No significant pairwise differences were found between racial groups in post hoc comparisons with Holm's adjustment. Bold denotes significant group differences at the 5% level.
HRSD, Hamilton Rating Scale for Depression; BGLAH, Brown–Goodwin Lifetime Aggression History scale.
Genotype, MDD diagnosis, childhood adversity and recent life stress
5-HT1A receptor genotype distribution at SNP rs6295 did not differ between the MDD and control groups (χ2 = 2.55, d.f. = 2, P = 0.2788) or between those with and without reported childhood adversity in the MDD group (χ2 = 3.59, d.f. = 2, P = 0.1665). In a linear model adjusted for age and gender, recent stress was not associated with genotype in the full sample (F = 0.07; d.f. = 2,214; P = 0.9365) or in the MDD group (F = 0.15; d.f. = 2,135; P = 0.8590) and diagnosis-related differences remained significant after adjustment for genotype, age and gender (F = 11.5; d.f. = 1,213, P = 0.0008).
Correlates of 5-HT1A receptor gene methylation
Methylation levels at −1019 and −1007 CpG sites were modestly positively correlated with each other (Spearman r s = 0.28, P < 0.0001), but neither correlated with methylation at the −681 CpG site (r s = 0.10, P = 0.1319; r s = 0.02, P = 0.7087).
Log-transformed methylation level measured at the −681 CpG site in a model with group and genotype as predictors, adjusting for age and gender, did not differ between groups (MDD versus control: b = −0.04, s.e. = 0.03, t = −1.16, d.f. = 272, P = 0.2479) and was unrelated to genotype (F = 0.76, d.f. = 2,272, P = 0.470). Methylation at −681 declined with age (b = −0.004, s.e. = 0.001, t = −3.35, d.f. = 272, P = 0.0009), but showed no gender differences (b = −0.01, s.e. = 0.03, t = −0.34, d.f. = 272, P = 0.7339). In the MDD group, −681 methylation was positively correlated with depression severity (Spearman r s = 0.18, P = 0.0194) but not with childhood adversity (b = 0.02, s.e. = 0.04, t = 0.42, d.f. = 164, P = 0.6776). Across all participants, −681 methylation was positively correlated with recent stress in a model adjusted for group, genotype, age and gender (b = 0.0001, s.e. = 0.0001, t = 2.01, d.f. = 212, P = 0.0457). The strength of association did not differ by group (interaction t = −0.28, d.f. = 211, P = 0.7782).
DNA methylation level at the −1007 CpG site did not differ between diagnostic groups or between genotypes, neither was there a correlation with recent stress (Supplementary Table 1 and Fig. 1). In the MDD group, −1007 methylation was not associated with childhood adversity (b = 0.03, s.e. = 0.04, t = 0.71, d.f. = 164, P = 0.478), neither was it correlated with depression severity (Spearman r s = −0.01, P = 0.8300).
DNA methylation at the −1019 CpG site (excluding the participants with GG genotype) did not differ by diagnostic group or correlate with recent stress (Supplementary Table 1 and Fig. 1). In the MDD group, adjusted for age and gender, methylation levels at the −1019 CpG was not associated with childhood adversity (b = 0.06, s.e. = 0.07, t = 0.94, d.f. = 134, P = 0.348), neither was there a correlation with depression severity (Spearman r s = −0.02, P = 0.6400).
5-HT1A binding potential: correlations with childhood adversity and recent stress, and group and genotype differences
In a model of the (log-transformed) [11C]WAY100635 BPF in all regions, with genotype, diagnosis, age and gender as independent variables, we confirmed our previous finding of higher binding in the MDD group compared with controls across all brain regions (b = 0.23, s.e. = 0.07, t = 3.37, d.f. = 113, P = 0.0010) but did not find a main effect for genotype (F = 1.22; d.f. = 2,113; P = 0.3000). There was evidence of significant differences between brain regions in the diagnosis effect (F = 5.22, d.f. = 12,1356, P < 0.0001) and even in the genotype effect (F = 4.36, d.f. = 24,1356, P < 0.0001), although region-wise post hoc analyses (Supplementary Table 2) did not identify any significant genotype effects.
In the MDD group, there was evidence of differential association between adversity and BPF by genotype and brain region (three-way interaction between region, childhood adversity and genotype F = 5.87; d.f. = 24,504; P < 0.0001). However, region-wise analyses did not find significant effect of childhood adversity or genotype effects on BPF in any individual region, either as main effects or through interaction (Supplementary Fig. 3).
In the full sample, when testing association of BPF with recent life stress, there was evidence of differential stress–BPF association by genotype and brain region (three-way interaction F = 3.88; d.f. = 24,1056; P < 0.0001). Diagnosis also moderated the recent stress–BPF association (three-way interaction F = 2.01, d.f. = 12,1080; P = 0.0210). To interpret these associations, separate analyses by diagnostic group and region were run to test the effect of stress on BPF and a possible moderation by genotype. Region-wise analyses in the MDD group found that recent stress had positive association with BPF in amygdala, orbital prefrontal cortex and insula (Table 2, Fig. 1, Supplementary Fig. 4) but no interactions were found between genotype and life stress, indicating that higher recent stress levels were associated with higher BPF in these regions, regardless of genotype.
Table 2 Region-wise associations between recent life change (RLCQ) and receptor binding potential (BPF)a

a. Separate analyses (adjusted for age and gender) are presented for participants with major depressive disorder and healthy volunteers. Outcome is log-transformed binding.
* Significant at the 5% level.
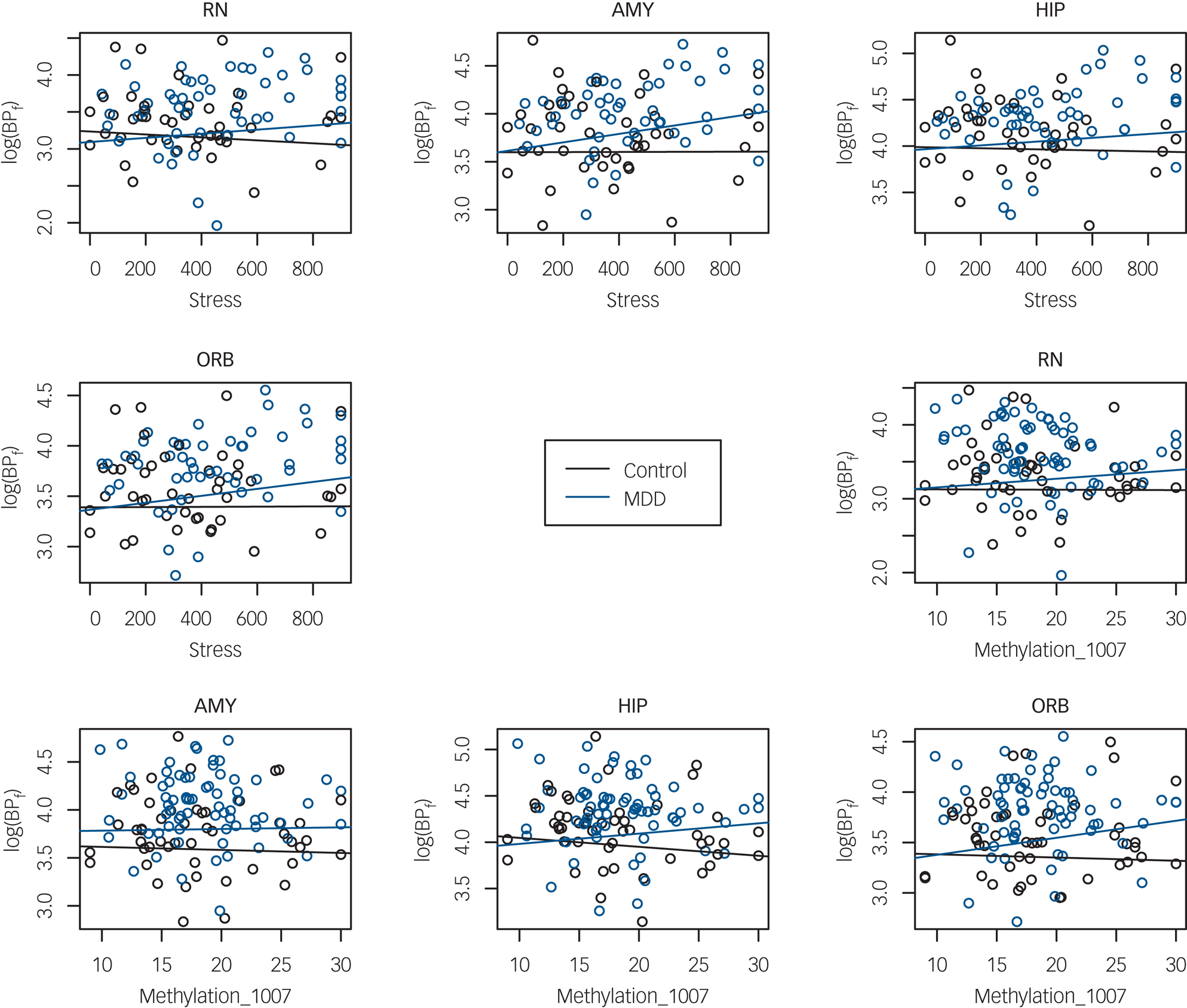
Fig. 1 Serotonin 1A receptor binding potential (BPf) in four brain regions as a function of recent life stress and of methylation level at the 5-HT1A promoter site −1007.
RN, raphe nuclei; AMY, amygdala; HIP, hippocampus; ORB, orbital cortex; MDD, major depressive disorder.
In contrast, in controls, no regions showed an association between recent stress and BPF; neither were there interaction effects with genotype (Table 2, Fig. 1, Supplementary Fig. 4).
5-HT1A binding potential and DNA methylation
In a model of BPF by methylation on the three sites and by diagnostic group, adjusted for genotype, age and gender, there was evidence of differential methylation effect by region and by group for two of the methylation sites (three-way interaction of region, diagnosis and methylation at the −1007 CpG site: F = 2.35; d.f. = 12,1044; P = 0.0057; and at the −681 CpG site: F = 6.17; d.f. = 12,1044; P < 0.0001). For the −1019 CpG site, the methylation–BPF association differed by region but not by diagnostic group (methylation × region interaction: F = 8.54; d.f. = 12,1044; P = <0.0001). Region-wise analyses in each group run separately revealed that participants with MDD, but not controls, displayed a positive correlation of DNA methylation at the −1007 CpG site with BPF in 7 of the 13 brain regions studied (raphe nuclei, hippocampus, cingulate, dorsolateral, medial prefrontal, orbital and parietal cortex); see Table 3 for numerical results, Fig. 1 for scatterplots of BPF correlation with DNA methylation at the −1007 CpG site in four brain regions, and Supplementary Figs 5–7 for pairwise scatterplots of BPF and DNA methylation level at each of the three sites in all brain regions.
Table 3 Effect sizes for region-wise associations between the three methylation sites and receptor binding potential (BPF)a
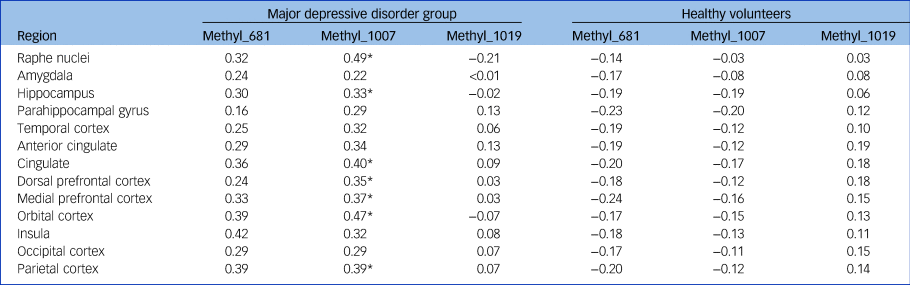
Methyl_, methylation level at the corresponding 5-HT1A promoter site (−681, −1007, −1019).
a. Separate analyses (adjusted for age, gender and genotype; sample excludes GG genotypes) are presented for participants with major depressive disorder and healthy volunteers. Outcome is log-transformed binding. Effect sizes are standardised estimates of (log-transformed) binding difference for 1 standard deviation difference in methylation level.
* Significant at the 5% level.
Supplementary Fig. 8 summarises all model findings.
Discussion
In this study, we report associations between recent stress and DNA methylation at the −681 CpG promoter site of the 5-HT1A receptor gene. Additionally, in participants with MDD but not in controls, positive correlations were found between DNA methylation at the −1007 CpG 5-HT1A receptor promoter site and higher 5-HT1A receptor binding potential and also between recent stress and binding potential. However, since the findings occurred in disparate CpG sites, mediation could not be tested formally.
Genotype, reported childhood adversity, recent stress and MDD
Our finding that a history of childhood adversity was essentially restricted to the MDD group is consistent with previous reports.Reference Moffitt, Caspi, Harrington, Milne, Melchior and Goldberg23,Reference Neigh, Gillespie and Nemeroff24 In addition, our findings of no association between 5-HT1A receptor promoter genotype and history of childhood adversity in the MDD group and no association with recent stress in either the full sample or the MDD group alone are consistent with a previous study in healthy volunteers.Reference Chipman, Jorm, Tan and Easteal25 Others report an association of the G-allele at the C(−1019)G site with greater stress reactivity, depression and suicide in adulthood.Reference Wasserman, Geijer, Sokolowski, Rozanov and Wasserman26–Reference Mekli, Payton, Miyajima, Platt, Thomas and Downey28
5-HT1A receptor DNA methylation, reported childhood adversity, recent life stress and MDD
Childhood adversity was not related to DNA methylation levels at the three promoter sites (−1019, −1007, −681) in peripheral blood monocytes in either the MDD group or controls; however, we cannot rule out DNA methylation effects of childhood adversity on the brain.
Recent stress was positively correlated with DNA methylation at the −681 CpG site, and this was not altered after controlling for group, genotype, age and gender. In contrast, DNA methylation at the −1019 and −1007 sites did not correlate with the −681 CpG or recent life stress. Similarly to our findings in humans, in stress-sensitive adult BALB/c mice, chronic mild unpredictable stress increased DNA methylation at the −681 CpG site.Reference Le Francois, Soo, Millar, Daigle, Le Guisquet and Leman10
5-HT1A receptor binding potential, MDD, DNA methylation and stress
We found that recent stress had a positive association with 5-HT1A receptor binding potential in the amygdala, orbital cortex and insula in the MDD group, consistent with the report that chronic adult stress increased 5-HT1A receptor RNA and receptor levels in both medial prefrontal cortex and midbrain in stress-sensitive BALB/c mice.Reference Le Francois, Soo, Millar, Daigle, Le Guisquet and Leman10 In the same study, increased 5-HT1A receptor binding was accompanied by an increase in DNA methylation at the −681 CpG site, which is consistent with our finding of a positive correlation of DNA methylation at the −681 CpG site with recent stress, although our findings are for peripheral methylation. However, in our study, only methylation at the −1007 site was positively correlated with PET binding in post hoc testing. The effects of peripheral DNA methylation at the −681 site appeared to be more variable among individual brain regions because of a complex three-way group × region × stressor interaction effect. This complexity meant that we could not posit a simple mediation model in which the effect of recent stress on 5-HT1A receptor binding is mediated through elevated DNA methylation levels at the −681 and/or −1007 CpG sites.
Limitations and strengths
A limitation of this study is that it was not possible to measure DNA methylation in brain in vivo, so we used peripheral blood monocyte DNA to assay methylation. Additionally, even though this is one of the largest PET studies of MDD, the numbers are still limited for an epigenetic study and we therefore did not adjust for multiple testing. We did not include participants with other Axis I disorders and did not adjust for comorbid axis II disorders such as borderline personality disorder. Our stress measurement method could not determine whether stress might be caused by the depressed state itself. Last, childhood adversity in this sample was almost exclusively reported in the MDD group and therefore its effect in controls could not be reported. Strengths of the study include the large sample of participants undergoing PET scans and that the MDD group were off antidepressant medication at the time of study.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2023.13.
Data availability
The de-identified data that support the findings of this study are available on reasonable request from the corresponding author (H.G.). The data are not publicly available because the participants in the protocol did not agree to share their data publicly.
Author contributions
All authors contributed to the writing of this paper and had the opportunity to revise the manuscript before submission. In addition, H.G. and J.J.M. contributed to formulating the research questions; J.J.M., J.M.M., M.A.O. and M.E.S. contributed to the design of the study; H.G. and E.S. analysed the data; J.M.M., F.Z. and S.P. contributed to the PET analysis, Y.H. analysed the genetic samples, A.K.B. conducted clinical evaluations and J.d.V. contributed to the conceptual model building.
Funding
This study was funded by National Institute of Mental Health (NIMH) awards MH090964 and MH040695.
Declaration of interest
J.J.M. and M.A.O. receive royalties for commercial use of the Columbia-Suicide Severity Rating Scale (C-SSRS) from the Research Foundation for Mental Hygiene. M.A.O.'s family owns stock in Bristol Myers Squibb. H.G. and her family own stocks in Illumina, Inc.










eLetters
No eLetters have been published for this article.