Similarities and differences between bipolar and borderline personality disorder
The relationship between borderline personality disorder (BPD) and bipolar disorder remains controversial.Reference Akiskal1, Reference Benazzi2 Both are severe mental disorders with a fluctuating course.3 Bipolar disorder is characterised by manic, depressive and mixed episodes, with intervals showing varying levels of euthymic remission.Reference Vieta, Berk, Schulze, Carvalho, Suppes and Calabrese4 BPD is defined as a long-standing pattern of functioning corresponding to a disorder of personality.Reference Akiskal5 However, longitudinal study shows fluctuation with remission of symptoms, including affective instability.Reference Akiskal, Chen, Davis, Puzantian, Kashgarian and Bolinger6, Reference Zanarini, Vujanovic, Parachini, Boulanger, Frankenburg and Hennen7 Pervasive problems in affect regulation have been considered the core feature of BPD by some researchers,Reference Coid8–Reference Linehan11 with rapid switching observed between different symptom profiles of anger, depression and anxiety.Reference Coid8, Reference Reisch, Ebner-Priemer, Tschacher, Bohus and Linehan12 Symptom profiles and course of affective disturbance appear to differ.Reference Henry, Mitropoulou, New, Koenigsberg, Silverman and Siever13, Reference Koenigsberg14
Clinical and community studies have shown high levels of diagnostic co-occurrence of the two disorders.Reference Gunderson, Weinberg, Daversa, Kueppenbender, Zanarini and Shea15–Reference Pereira, Köhler, De Sousa, Solmi, De Freitas and Fornaro17 A diagnosis of BPD in young people can precede a diagnosis of bipolar disorder in adulthood.Reference McDermid, Sareen, El-Gabalawy, Pagura, Spiwak and Enns18, Reference Yen, Frazier, Hower, Weinstock, Topor and Hunt19 It has also been suggested that BPD and bipolar disorder are alternative expressions of the same disorder,Reference de la Rosa, Oquendo, García, Stanley, González-Pinto and Liu20 and that BPD is on a bipolar spectrum of affective disorder,Reference Akiskal1, Reference Benazzi2, Reference Akiskal, Chen, Davis, Puzantian, Kashgarian and Bolinger6, Reference Walsh, DeGeorge, Barrantes-Vidal and Kwapil21 characterised by ultra-rapid-cycling.Reference Akiskal5 It has been suggested that rapid switching and the affective instability of BPD could originate from similar genetic aetiology as bipolar disorder. However, mood-stabilising medication is considered the key treatment intervention for bipolar disorder, whereas psychotherapy is the key treatment for BPD.Reference Ruggero, Zimmerman, Chelminski and Young22, Reference Gunderson and Phillips23 Furthermore, although the two conditions can co-occur, it is argued that their course, family history and treatment response are very different, and that misdiagnosis can result in patients not receiving appropriate treatment.Reference Paris and Black24
Neuroimaging studies of the two disorders
In the absence of a definitive understanding of the neuropathology underpinning these two disorders, there are currently no clinical biomarkers available to aid diagnosis, clarify the relationship between bipolar disorder and BPD or indicate the most appropriate treatment. Biomarker discovery and optimization are therefore essential. Neuroimaging studies have been used to identify differences between patients with affective disorders and healthy controls in structural magnetic resonance imaging (MRI) analysis.Reference Wise, Radua, Via, Cardoner, Abe and Adams25, Reference Ganzola and Duchesne26 Changes in grey matter volume (GMV) and grey matter density (GMD) in BPD have been most consistently found in the amygdala and hippocampus;Reference Nunes, Wenzel, Borges, Porto, Caminha and de Oliveira27, Reference Yang, Hu, Zeng, Tan and Cheng28 brain abnormalities in bipolar disorder are frequently reported within the frontal-striatal-limbic network.Reference Pereira, Köhler, De Sousa, Solmi, De Freitas and Fornaro17, Reference Wise, Radua, Via, Cardoner, Abe and Adams25 However, it is unclear whether changes in brain structure can differentiate BPD from bipolar disorder, or whether common abnormalities are present in both disorders.Reference McDermid, Sareen, El-Gabalawy, Pagura, Spiwak and Enns18 Only one study has directly compared BPD and bipolar disorder, which found that GMD changes in bipolar disorder were more diffuse and severe than in BPD, but had certain regions of overlap.Reference Rossi, Pievani, Lorenzi, Boccardi, Beneduce and Bignotti29 To date, small sample sizes, heterogeneity and differences in analytical method (e.g. whole-brain or region-of-interest (ROI) analyses) have resulted in inconsistency. A meta-analysis may provide a more precise evaluation of potential diagnostic biomarkers at the brain level, clarify the uncertain relationship between bipolar disorder and BPD and ultimately improve classification.Reference Kempton, Salvador, Munafò, Geddes, Simmons and Frangou30
The aim of this meta-analysis was to establish the most consistent brain function abnormalities of bipolar disorder and BPD, using all published, whole-brain structural MRI studies that do not bias findings to a priori hypothesised regions.Reference Lim, Radua and Rubia31
Method
Searches and study selection
We conducted a literature search of PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), EMBASE (www.embase.com), Google Scholar (http://scholar.google.com/) and Science Direct (https://www.sciencedirect.com/) for voxel-based morphometry (VBM) studies published between 1 January 2003 and 28 February 2018. In addition, we conducted manual searches among the reference sections of all retrieved studies and review articles.Reference Norman, Carlisi, Lukito, Hart, Mataix-Cols and Radua32 To ensure comprehensiveness, two researchers performed the searches independently, using keywords such as ‘borderline personality’, ‘bipolar/affective/mood disorder’, ‘mania/hypomania/bipolar depression/’, ‘neuroimaging’, ‘mri’, ‘magnetic resonance’, ‘brain imaging’, ‘morphometry’ and ‘voxel’. We followed Preferred Reporting Items For Systematic Reviews and Meta-analyses (http://www.prisma-statement.org) (Fig. 1) guidelines for meta-analyses of observational studies.Reference Modinos, Costafreda, van Tol, McGuire, Aleman and Allen33
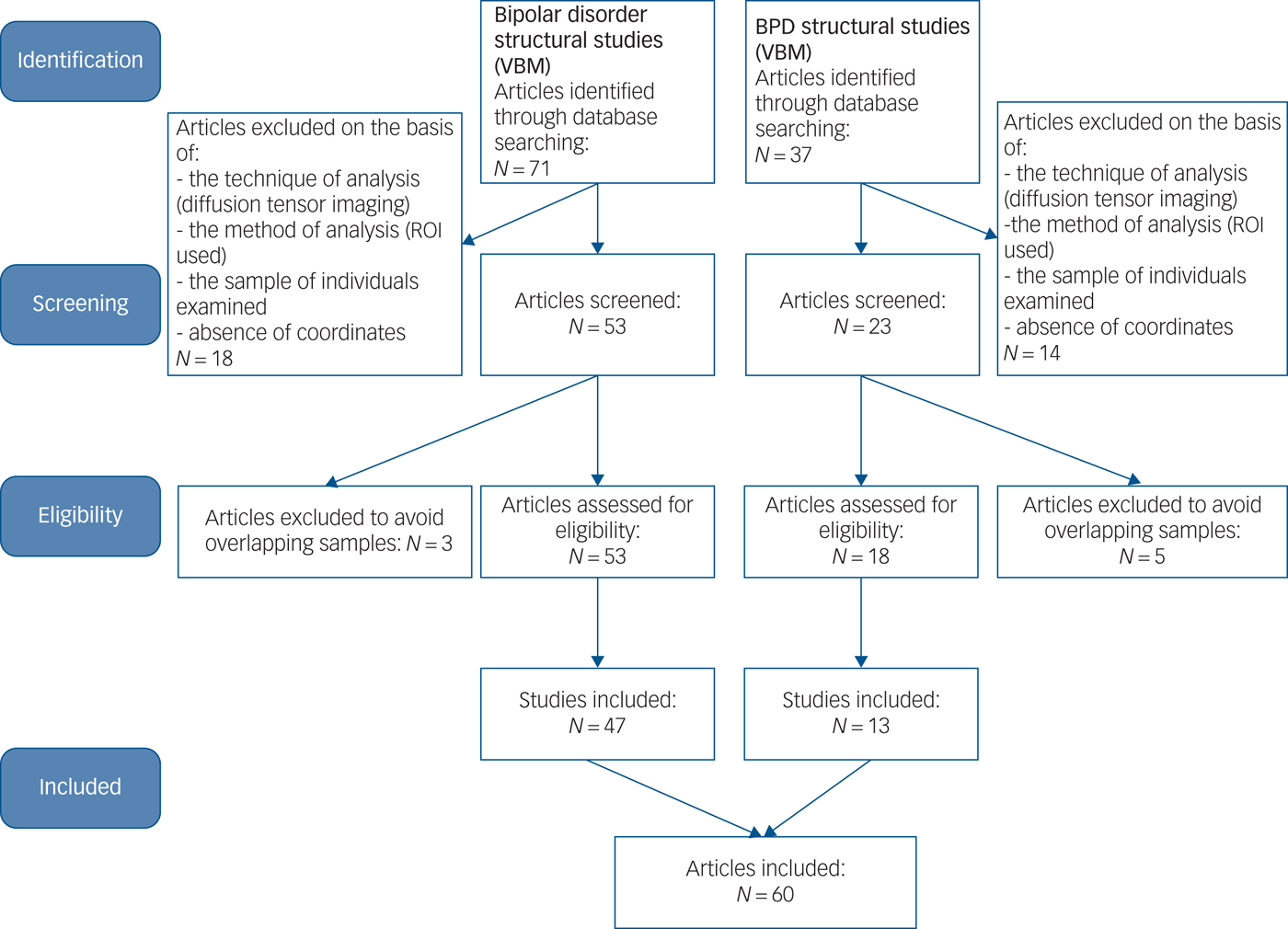
Fig. 1 A Preferred Reporting Items For Systematic Reviews and Meta-analyses (PRISMA) flowchart of the article selection. PRISMA flowchart for the meta-analysis of voxel-based morphometry (VBM) studies in patients with bipolar disorder and borderline personality disorder (BPD). ROI, region of interest.
Our inclusion criteria were as follows: (a) the study included patients with BPD versus healthy controls, and patients with bipolar disorder versus healthy controls; (b) the VBM method was used to analyse whole-brain grey matter changes of patients with bipolar disorder and patients with BPD; (c) the study reported peak coordinates of the brain areas as well as the statistical parametric maps, and coordinates were normalised into a stereotaxic standardised space (e.g. the Montreal Neurological Institute (MNI) or Talairach space).Reference Bora, Fornito, Yücel and Pantelis34 Authors of published reports were contacted by email when required information was not provided.
Our exclusion criteria were as follows: (a) neuroimaging techniques other than MRI whole-brain VBM (using ROI/voxel-of-interest methods might result in selection bias); (b) participants aged <18 years or >65 years (to minimise effects of neurodevelopment and neurodegeneration); (c) patients diagnosed with schizoaffective disorders or any comorbid neurological conditions; (d) review papers or meta-analysis studies; and (e) when t- or z-maps were unavailable, consistent statistical thresholds throughout the brain were not used or peak coordinates were not reported.Reference Wise, Radua, Via, Cardoner, Abe and Adams25 For samples shared with other studies, the study with the largest sample was included.Reference Wise, Radua, Via, Cardoner, Abe and Adams25 Conversely, when the same control group was used in several subgroup comparison, only a combined summary result was included in the meta-analysis.Reference Schulze, Schmahl and Niedtfeld35 In the BPD group, we specifically excluded samples with co-occurring bipolar disorder, and vice versa.Reference Bruno, Barker, Cercignani, Symms and Ron36 For studies that used longitudinal treatment designs, only baseline pre-treatment data were included. Using this approach, we selected a pool of 13 BPD studies and 47 bipolar disorder studies.
Recorded variables and contrasts
The following variables were recorded from each article: sample size, mean age of participants, gender (male/female), mean illness duration, mean scores of Young Mania Rating Scale (YMRS) and Hamilton Rating Scale for Depression (HRSD) for bipolar disorder, proportion medicated for bipolar disorder, bipolar disorder subtype (1 or 2), magnetic field strength and image analysis tools (shown in Supplementary Tables 1 and 2 available at https://doi.org/10.1192/bjp.2019.44). Coordinates with statistically significant differences were extracted, including direction of alteration (BPD/bipolar disorder > healthy controls, BPD/bipolar disorder < healthy controls). Coordinates in different stereotactic spaces were automatically converted by SDM software and Z- or P-values for significant clusters were converted to t-statistics by SDM online conversion utilities (https://www.sdmproject.com/utilities/?show=Statistics).
Meta-analysis
Regional differences in GMV and GMD between patients with BPD or bipolar disorder and healthy controls were analysed using the effect-size version of signed differential mapping (AES-SDM; http://www.sdmproject.com/).Reference Radua, Rubia, Canales-Rodríguez, Pomarol-Clotet, Fusar-Poli and Mataix-Cols37 Voxel-based meta-analytic methods have been described in detail elsewhere.Reference Nakao, Radua, Rubia and Mataix-Cols38 In brief, the main features of our method (AES-SDM) included extraction of peak coordinates and effect sizes of grey matter differences between patients with BPD or bipolar disorder and healthy controls from each data-set,Reference Benazzi2, Reference Hart, Radua, Nakao, Mataix-Cols and Rubia39 recreation of a map of the effect sizes of the differences between patients and controls for each study and a standard random-effects variance-weighted meta-analysis for each voxel. Default AES-SDM kernel size and thresholds were used (full width at half maximum = 20 mm, voxel P = 0.005, peak height Z = 1, cluster extent of 10 voxels).3, Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg and McGuire40 We performed the meta-analysis on the weighted mean difference in regional grey matter between patients and healthy controls, weighted by the square root of the sample size of each study, so that studies with large sample sizes contributed more;Reference Vieta, Berk, Schulze, Carvalho, Suppes and Calabrese4, Reference Radua, Rubia, Canales-Rodríguez, Pomarol-Clotet, Fusar-Poli and Mataix-Cols37 we confirmed reliability by checking the results;Reference Akiskal5 Jackknife sensitivity analyses were conducted to establish reproducibility of results by iteratively repeating analyses, excluding one data-set each time.Reference Radua and Mataix-Cols41
The results replicated in at least 70% of the studies were reliable. Funnel plots and Egger tests were conducted to identify conflicting studies and publication bias.Reference Carlisi, Norman, Lukito, Radua, Mataix-cols and Rubia42 A P-value <0.05 was considered significant.Reference Pan, Zhan, Xia, Zhang, Guan and Xu43 Finally, we used the multi-modal analysis function of the AES-SDM statistical package, which allowed us to perform conjunction analysis. This enabled us to compare abnormalities between conditions (BPD versus bipolar disorder) based on evaluation of effect sizes to identify regions where both patient groups showed shared differences with respect to controls, as well as taking into account errors in the estimation of magnitude of these differences.Reference Wise, Radua, Via, Cardoner, Abe and Adams25
Meta-regression analysis
The potential effect of several relevant sociodemographic and clinical variables were examined by linear regression, weighted by the square root of the sample size and restricted to only predict possible SDM values.Reference Radua and Mataix-Cols41 For bipolar disorder, we used depressive symptoms, manic symptoms, illness duration, mean patient age and female patient percentage. Given the relatively small number of studies, we set the cut-off value for inclusion of potential confounders in meta-regressions to ≥20 studies for bipolar disorder to minimise occurrence of false positive values.Reference Higgins and Thompson44 For BPD, we used mean patient age and female patient percentage. We also examined effects of magnetic field strength and image smoothing level for both conditions. Studies that did not report these measures were excluded from analyses.Reference Wise, Radua, Via, Cardoner, Abe and Adams25
Results
General information for each sample group
The 13 structural MRI studies on BPD included 395 patients with BPD and 415 healthy controls (Supplementary Table 1). Their mean age was 29.1 years, 336 (75.3%) were female, 63 (15.95%) had co-occurring major depressive disorder and 94 (23.80%) had post-traumatic stress disorder. The 47 bipolar disorder studies included 2111 patients and 3261 healthy controls (Supplementary Table 2). Their mean age was 34.4 years, 1119 (53.01%) were female, 24 studies reported YMRS scores with mean score of 8.0 and 25 studies reported HRSD scores with mean scores of 8.63. The bipolar disorder cohorts included 1690 (80.06%) patients with bipolar disorder type 1, 252 (11.94%) patients with bipolar disorder type 2 and 176 (8%) patients with other bipolar disorder types. The mean illness duration for bipolar disorder was 14.36 years (30 studies reported). Patients with BPD were significantly younger than patients with bipolar disorder (omnibus test P < 0.001, 5.32 years) and there were more females patients with BPD (omnibus test P < 0.001, 23% female).
Meta-analysis
BPD versus healthy controls
Patients with BPD showed significantly decreased GMV and GMD in the bilateral medial prefrontal cortex (mPFC), medial orbital frontal cortex (OFC), bilateral anterior cingulate cortex (ACC), bilateral amygdale and right parahippocampal gyrus compared with healthy controls. They also showed increased GMV and GMD in the bilateral praecuneus, right medium / paracingulate gyrus (Brodmann area (BA) 23) and posterior cingulate gyrus (BA 23) compared with healthy controls (Table 1 and Fig. 2a). Jackknife sensitivity analyses revealed that observed deficits in bilateral posterior cingulate gyrus, bilateral mPFC, medial OFC and ACC were highly robust and were replicable in all 13 studies. Deficits in bilateral amygdala and right parahippocampal gyrus were replicable in 10 out of 13 studies.
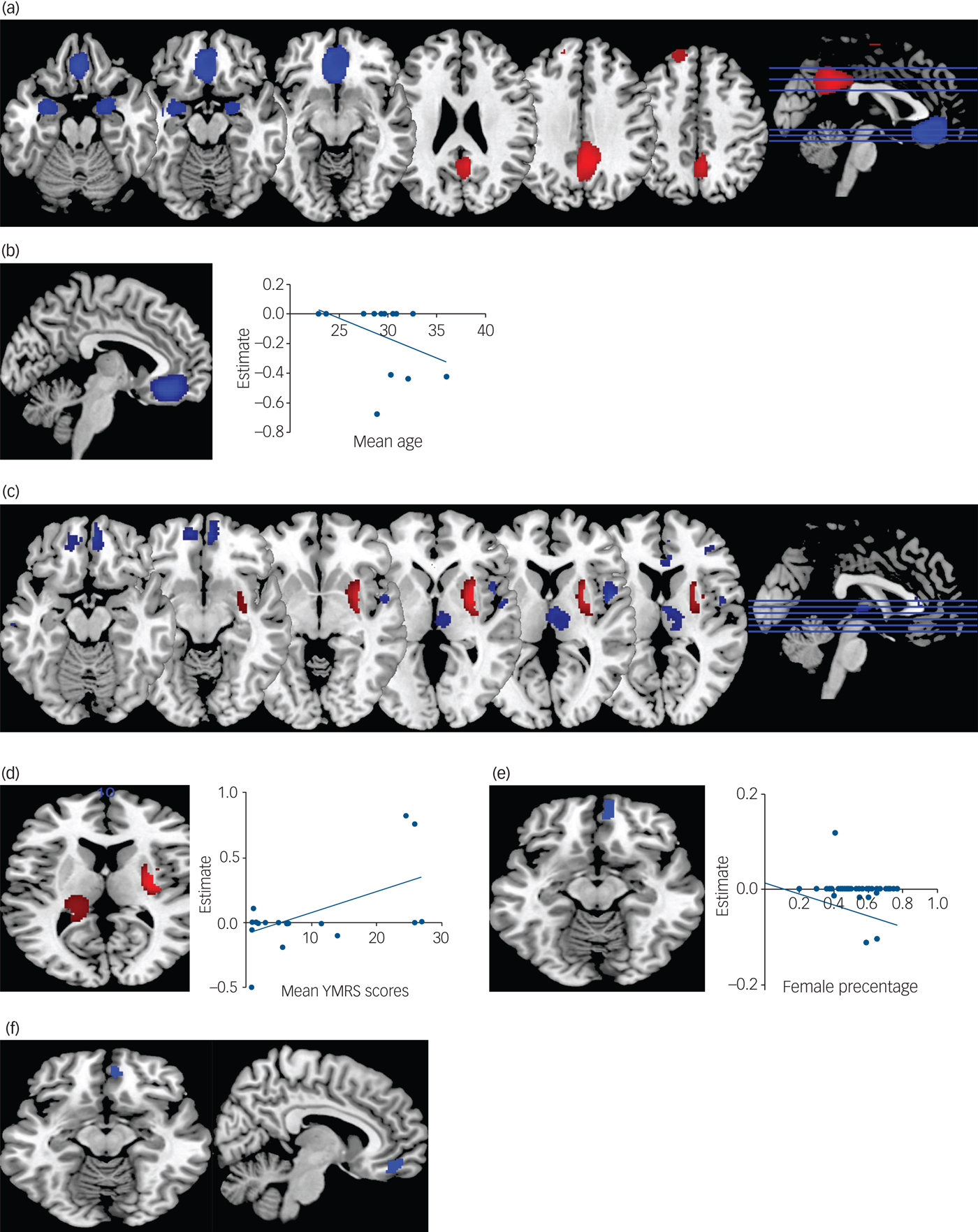
Fig. 2 Meta-analysis results. (a) Results of borderline personality disorder (BPD) meta-analysis. (b) Results of meta-regression with age in BPD. (c) Results of bipolar disorder meta-analysis. (d) Results of meta-regressions with Young Manic Rating Scale (YMRS) scores in bipolar disorder. (e) Results of meta-regression with gender in bipolar disorder. (f) Results of the conjunction analysis showing regions with similar volumetric alterations in both conditions. Blue represents lower volume in both conditions relative to controls; red represents greater volume relative to controls or negative relationships with regressors. In meta-regression plots, point size represents study weights. All images are shown in neurological convention; the left of the image corresponds to left of the brain. Effect sizes represent effect sizes at the peak of the cluster.
Table 1 Regional differences in grey matter volume between individuals with BPD and healthy controls and individuals with bipolar disorder and healthy controls
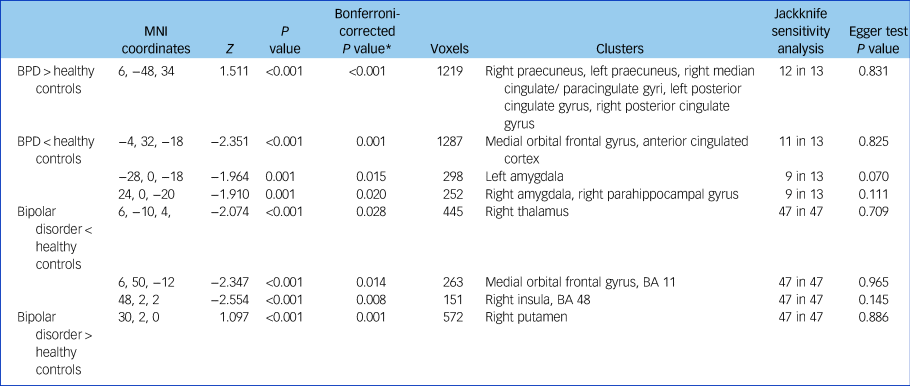
BPD, borderline personality disorder; MNI, Montreal Neurological Institute; BA, Brodmann area.
* P values were corrected by multiplying the contrasts number with the uncorrected P values.
Meta-regression analyses revealed that smaller volumes relative to controls were associated with increasing patient age in the right orbital frontal gyrus (OFC) (Fig. 2b; peak MNI = −6, 34, −14; Z = −3.526; P < 0.001; 1401 voxels). When examining the methodological variables in the BPD group, studies using higher field strength scanners and higher full width at half maximum showed greater GMV and GMD relative to controls in the right praecuneus (peak MNI = 12, −38, 33; Z = 1.352; P < 0.001; 189 voxels; peak MNI = 6, −58, 34; Z = 1.687; P < 0.001; 541 voxels).
Bipolar disorder versus healthy controls
Several reduced GMD and GMV clusters were identified in patients with bipolar disorder compared with healthy controls, including decreased GMV and GMD in the bilateral medial OFC, right insula and right thalamus, whereas increased GMV and GMD was found in the right putamen (Table 1 and Fig. 2c). Whole-brain jackknife sensitivity analysis revealed that the GMV and GMV decrease in the aforementioned brain areas was highly replicable. This finding was observed in all 47 studies and analyses. Meta-regression analyses revealed that higher YMRS scores were associated with greater GMV and GMD volumes compared with controls in the right putamen (Fig. 2e; peak MNI = 32, −8, 6; Z = 2.966; P < 0.001; 684 voxels). We also found smaller volumes in the right OFC relative to controls were associated with an increasing percentage of female patients (Fig. 2d; peak MNI = 6, 62, −12; Z = −2.93; P < 0.001; 106 voxels). We found no significant associations between HRSD scores, patient age, mood state, lithium use and methodological variables, and abnormal brain findings in bipolar disorder.
BPD versus bipolar disorder
Conjunction analysis indicated that brain regions in the right medial OFC showed smaller volume compared with controls in both conditions (Fig. 2f; peak MNI = 0, 34, −20; P < 0.001; 417 voxels). No regions showed greater volume. Because of small sample sizes directly comparing patients with bipolar disorder and patients with BPD,Reference Rossi, Pievani, Lorenzi, Boccardi, Beneduce and Bignotti29 we could not contrast the BPD and bipolar disorder group in subgroup analyses.
Analyses of publication bias
Egger tests for publication bias were not significant with respect to grey matter differences in all study results. All P values were >0.05 (Table 1).
Discussion
To our knowledge, this study is the first voxel-based meta-analysis to investigate differences between individuals with BPD and individuals with bipolar disorder in neuroimaging studies across the entire brain. We compared results to identify both common and different patterns of GMV alterations. We found that the two conditions were substantially different and shared similar patterns of lower volume only in the right medial OFC. However, decreased volume of OFC was partly mediated by patient age in the BPD group. Decreased GMD and GMV of the bilateral amygdala and right parahippocampal gyrus were consistently found in BPD.Reference Yang, Hu, Zeng, Tan and Cheng28, Reference Ruocco, Amirthavasagam and Zakzanis45 In contrast, the right insula, right thalamus and right putamen were differently affected in bipolar disorder. Our results indicate that the two disorders are associated with spatially distinct patterns of brain structure.
Overview of emotion dysregulation theory in BPD versus bipolar disorder
As emotion dysregulation is a core feature of both bipolar disorder and BPD, it can make these two disorders often indistinguishable.Reference Coulston, Tanious, Mulder, Porter and Malhi46 However, differing emotion regulation profiles were observed between the disorders, with emotional dysregulation in those with BPD generally considered to be reactive and precipitated by stressors or interpersonal difficulties,Reference Renaud, Corbalan and Beaulieu47 possibly reflecting behavioural sensitisation (in which repeated exposure to a particular stressor, such as ongoing abuse or neglect, evokes strong reactions).Reference Post and Weiss48 In bipolar disorder, emotion dysregulation is considered to be more autonomous and internally driven, and also less reactive to psychosocial cues.Reference Renaud, Corbalan and Beaulieu47 Although cognitive and emotion domains are typically studied independently, basic research and emergent findings in bipolar disorder suggest that there are important ties between cognitive deficits and the emotion disturbances observed in bipolar disorder.Reference Lima, Peckham and Johnson49 A large body of behavioural and neural studies suggests that effective emotion regulation rests on strong executive function, and particularly inhibition.Reference Green, Cahill and Malhi50 In accordance with previous BPD and bipolar disorder literature review, our structural neuroimaging results are quite in consistent with the different emotion regulation strategies described above, and we discuss them in detail below.
BPD-specific brain volume abnormality
Disturbed interpersonal relationships and affect regulation are fundamental aspects of BPD.Reference Zanarini, Frankenburg, Reich, Silk, Hudson and McSweeney51 The amygdala plays important roles in modulating vigilance and generating negative emotional states and is often abnormally reactive in BPD.Reference Niedtfeld, Schulze, Krause-Utz, Demirakca, Bohus and Schmahl52 Compared with healthy controls, patients with BPD showed greater amygdala activation, particularly in response to repeated emotional stimuli, and a prolonged return to baseline for overall blood oxygen level–dependent response averaged across all pictures.Reference Donegan, Sanislow, Blumberg, Fulbright, Lacadie and Skudlarski53 The decreased amygdala volume here we found might be the structural basis for the functional abnormalities as previously described by O'Neill and Frodl.Reference O'Neill and Frodl54 The adjacent parahippocampal gyrus projects to the hippocampus and the limbic circuit, and plays a role in memory encoding and retrieval, especially with regard to retrieving information about familiarity of scenes. It also plays a role in identifying sarcasm in verbal communication.Reference Soloff, Pruitt, Sharma, Radwan, White and Diwadkar55 Retrieval of specific and positive autobiographical memories, a function of the hippocampus, is impaired in BPD and is related to poor problem-solving.Reference Rankin, Salazar, Gorno-Tempini, Sollberger, Wilson and Pavlic56 During an emotional crisis, this associative memory function enables a person to envision positive outcomes based on past experiences.Reference Mark and Broadbent57 The subcortical area abnormalities we found in BPD are quite inconsistent with the previous published meta-analysis. In 2012, Ruocco et al Reference Ruocco, Amirthavasagam and Zakzanis45 reported that the bilateral volumes of amygdala and hippocampus were modestly reduced in BPD, which cannot be attributed to illness state or comorbid psychopathology.
Studies in healthy controls suggested intact coupling between prefrontal cortex, particularly the ventral prefrontal cortex, and amygdala, the parahippocampal gyrus may be the neural substrate for downregulation of the amygdala in response to aversive stimuli.Reference Tebartz van Elst, Hesslinger, Thiel, Geiger, Haegele and Lemieux58 The absence of such close coupling in patients with BPD, indicated by the lack of significant correlations between the OFC and amygdala/parahippocampal gyrus, suggests a disconnect between the OFC and limbic areas, which may explain the failure of patients with BPD to downregulate the subcortical areas in response to aversive stimuli.Reference New, Hazlett, Buchsbaum, Goodman, Mitelman and Newmark59 It is likely that the mPFC volume deficit is partly mediated by age and that the affected higher-order brain areas are not as pervasive as in bipolar disorder. The less-affected higher-order cortical cortex might explain why patients with BPD benefit from treatments targeting emotion recognition.Reference Hazlett, Zhang, New, Zelmanova, Goldstein and Haznedar60
Bipolar disorder–related brain volume abnormality
Our results are consistent with previous studies performed in patients with bipolar disorder and affective disorders.Reference Wise, Radua, Via, Cardoner, Abe and Adams25, Reference Phillips, Ladouceur and Drevets61 Wise et al reported abnormal GMV changes in mPFC systems, the insula and limbic areas in bipolar disorder,Reference Wise, Radua, Via, Cardoner, Abe and Adams25 and Phillips et al proposed the theory that in mood dysregulation in affective disorders, differences in the brain regions amygdala, insula, ventral striatum, ACC and prefrontal cortex may lead to the aberrant mood states.Reference Phillips, Ladouceur and Drevets61 The insular cortex has recently become an area of focus in psychiatric research, particularly the anterior insula cortex (AIC). This region is involved in a range of functions, including affective processing and awareness of bodily states.Reference Li, Tang, Womer, Fan, Zhou and Sun62, Reference Lochhead, Parsey R, Oquendo and Mann63 Atypical functioning of this region in affective disorders has been found in functional neuroimaging research and convergent evidence indicates insular abnormalities in bipolar disorder.Reference Brooks, Wang, Bonner, Rosen, Hoblyn and Hill64, Reference Bechdolf, Wood, Nelson, Velakoulis, Yücel and Takahashi65 Our finding of smaller insular volume in patients with bipolar disorder suggests that structural abnormalities are present in the same areas in which altered function has also been identified previously, and may imply impairment of insula functioning, leading to abnormal cognitive and visceral responses to negative emotional stimuli.Reference Bush, Luu and Posner66
The thalamus serves as a relay station within forebrain circuits to the cerebral cortex and to limbic structures.Reference Jones67 It has been increasingly implicated in emotional and cognitive processing, particularly via cortico-basal ganglia and cortico-thalamic circuits, and appears to play important roles in executive functions that are commonly impaired in psychotic conditions.Reference Womer, Wang, Alpert, Smith, Csernansky and Barch68 Functional connectivity data have also shown functional connections between the thalamus and anterior insula,Reference Chai, Whitfield-Gabrieli, Shinn, Gabrieli, Nieto Castañón and McCarthy69 and the connections between the AIC and thalamus suggest that the AIC is involved in modulation of thalamic function. Our results further confirm previous studies reporting that thalamic dysfunction is combined with insula abnormalities, which is associated with aberrant emotional, cognitive and social behaviour in bipolar disorder.Reference Strakowski, Delbello and Adler70 Our meta-analysis showed increased GMD and GMV of the striatum and is positively correlated with YMRS scores. Therefore, increased volume of putamen is very likely a state effect. The results are also consistent with clinical observations that patients with bipolar disorder have enhanced reward intensity.Reference Nusslock, Almeida, Forbes, Versace, Frank and Labarbara71
We also found decreased GMV and GMD of OFC in bipolar disorder. Rodent and primate studies indicate that the OFC is involved in the assessment of emotional significance and reward potential of stimuli, and regulates the amygdala.Reference Nusslock, Almeida, Forbes, Versace, Frank and Labarbara71, Reference Öngür and Price72 Abnormalities in this system can cause an inability to assess emotional significance of stimuli, leading to inappropriate emotional responses and impulsive behaviour.Reference Antoniadis, Samakouri and Livaditis73 In our results, we found decreased OFC volume in patients with bipolar disorder, which was more severe in female patients, consistent with previous evidence showing that female patients with bipolar disorder report higher levels of both positive and negative affect over time.Reference Diener, Sandvik and Larsen74 Our data partially support the hypothesis that mood disorder represents a decrease in cortical activation and increase in limbic activation, but also highlights a more complex and nuanced picture that differs across mood states/traits and cortical subregions.
Common affected medial OFC in BPD and bipolar disorder
Our finding of OFC deficits in both clinical groups may indicate that these changes are not BPD-specific but rather imply a general biological vulnerability to the development of psychiatric disturbances.Reference Brunner, Henze, Parzer, Kramer, Feigl and Lutz75 For instance, deficits in OFC areas are shared with other psychiatric disorders like depressionReference Öngür and Price72, Reference Murray, Wise and Drevets76 and addiction disorders.Reference Volkow, Wang, Ma, Fowler, Wong and Ding77, Reference Kantak, Yager and Brisotti78 Thus, it may be hypothesised that BPD could be a frontal-deficit spectrum disease that shares frontal deficits with disorders other than bipolar disorder. Our finding of prefrontal deficits may be less of an endophenotypic pattern for a specific diagnosis, but may instead represent a pattern that reflects dysfunctioning of cognitive abilities (like attention, working memory and declarative memory), which impairs the capacity to control emotions (129,130). However, further longitudinal studies that over the time course of the disease are needed to verify whether the pattern of changes in brain morphology is unique for BPD. Longitudinal development in BPD should then be compared with longitudinal developments in other disorders.Reference Brunner, Henze, Parzer, Kramer, Feigl and Lutz75
Limitations
There are several limitations to this study. The first limitation comes from the SDM VBM method, as it was based on peak coordinates and effect sizes from published studies rather than raw statistical brain maps, which may result in less accurate findings.Reference Lim, Radua and Rubia31 To date, there have been few VBM studies directly comparing bipolar disorder and BPD, making it difficult to draw definitive conclusions on similarities and differences. In addition, by using the SDM VBM method we cannot exclude the possibility that our finding of abnormal subcortical areas (i.e. abnormal parahippocampal gyrus and amygdala volumes in BPD) but no differences in amygdala volume in bipolar disorder may be due to the edge effects or reduced sensitivity of whole-brain VBM analyses in small regions, such as the hippocampus and amygdala.Reference Bergouignan, Chupin, Czechowska, Kinkingnéhun, Lemogne and Le Bastard79 Manual segmentation remains the gold standard for analysing subcortical structures like the hippocampus and the amygdala. However, this method has limitations, such as obtaining each ROI in the native space without image transformation, as well as being operator-dependent and highly time-consuming.Reference Grimm, Pohlack, Cacciaglia, Winkelmann, Plichta and Demirakca80 Other popular method-automatic segmentation, such as the ROI-based approach by FreeSurfer, is increasingly accepted. Even for small regions, entirely automated use of FreeSurfer is acceptable in large data-sets. However, the approach used in this meta-analysis, although indirectly comparing studies’ effect sizes, offers the most viable option that allows for conclusions generalisable beyond individual studies. Furthermore, given the robustness of the meta-analytic method, our results provide a summary of the most reliable differences observed between bipolar disorder and BPD.
The second limitation is the lack of heterogeneity analysis across the patient populations. Clinical differentiation is seemingly more difficult in differentiating BPD from the bipolar disorder type 2 subtype.Reference Fletcher, Parker, Bayes, Paterson and McClure81 However, within the studies included in this analysis, only one included the bipolar disorder type 2 samples in their analysis,Reference Narita, Suda, Takei, Aoyama, Majima and Kameyama82 so we cannot perform a subgroup analysis directly by dividing the bipolar disorder group into type 1 and 2 subtypes. Furthermore, the prevalence of psychosis in BPD and bipolar disorder groups could significantly contribute to the differences reported in this study because of the comorbidity between psychosis and both groups.Reference Bassett83 Further studies should be performed using subgroup analysis considering the issues of comorbidities.
Third, we cannot determine causality from these results because all studies were cross-sectional group comparisons. It is unclear whether these alterations are part of the genetic pathogenesis of these disorders or a consequence of illness.Reference Wise, Radua, Via, Cardoner, Abe and Adams25 In addition, given that psychotropic medications and psychotherapy can have demonstrable effects on brain structure,Reference Zanarini, Frankenburg, Hennen and Silk84, Reference Arnone, McKie, Elliott, Juhasz, Thomas and Downey85 it is difficult to be certain that results are not entirely independent from treatment status. To solve these issues, longitudinal and clinical trials focusing on treatment effects are needed in future studies.
Finally, several studies reported GMD rather than volume. The mean GMD is derived from the percentage of absolute GMV divided by total brain volume. This might result in different locations of deficit areas from results achieved by VBM measurements of GMV.Reference Wang, Yu, Liu, Yin, Wang and Wang86
In conclusion, BPD and bipolar disorder show distinct patterns of GMV abnormalities in a number of regions. Differences were predominantly observed in the amygdala and parahippocampal gyrus in BPD and the cortical-thalamic-striatal circuit in bipolar disorder. Although there is significant heterogeneity within these results, this was partially explained by clinical and demographic differences in clinical samples. These findings suggest new targets for neuroanatomical diagnostic biomarkers. They do not suggest that BPD falls within the bipolar spectrum.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.44.
Acknowledgements
This work was partly funded by the National Key Research and Development Program of the Ministry of Science and Technology of China (grant 2016YFC0904300 to T.L.), the National Nature Science Foundation of China Key Project (grants 91332205, 81630030 and 81130024 to T.L.), the National Nature Science Foundation of China Project (grant 81601172 to Y.M., 81501174 to M.L.L. and 81681571305 to W.G.), the National Natural Science Foundation of China/Research Grants Council of Hong Kong Joint Research Scheme (grant 81461168029 to T.L.) and the 1.3.5 Project for disciplines of excellence, West China Hospital of Sichuan University (grants ZY2016103 and ZY2016203 to T.L.).








eLetters
No eLetters have been published for this article.