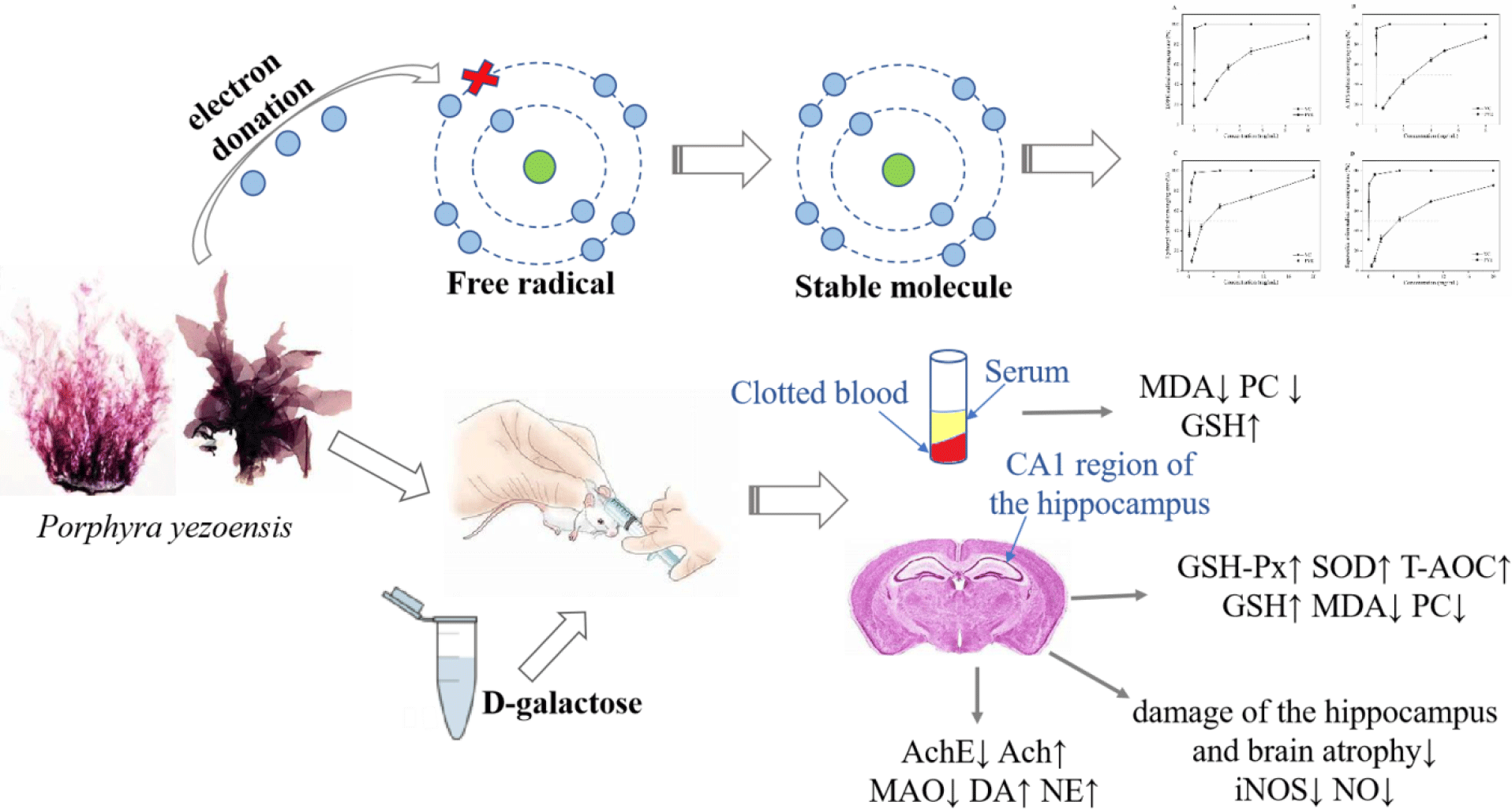
Harman outlined a theory on the mechanisms of ageing based on free radical chemistry: ‘Ageing and the degenerative disease associated with it are attributed basically to the deleterious side attacks of free radicals on cell constituents and on the connective tissues’(Reference Harman1). There is a growing body of evidence that reactive oxygen species (ROS) modify biological molecules, such as lipids and proteins, leading to impaired cellular function, including neuronal death in the hippocampus(Reference Kou, Zhu and Yan2,Reference Rehman, Shah and Ali3) . Oxidative stress is considered to play a pivotal role in the normal ageing processes and in neural loss in various neurodegenerative diseases, such as Alzheimer’s disease(Reference Tu, Chang and Chou4,Reference Xu, Xie and Gui5) . Anti-ageing and neurodegenerative diseases treatments have attracted extensive attention worldwide.
d-Galactose (d-gal) is a naturally occurring chemical substance in the body. However, high doses of d-gal lead to the accumulation of galactitol, resulting in osmotic stress and ROS production(Reference Haider, Liaquat and Shahzad6). In addition, accumulated d-gal can react with the amino groups of proteins and peptides to form advanced glycation end products, which have been linked to neuronal cell death in many age-related neurodegenerative diseases(Reference Chen, Lang and Zuo7,Reference Hsieh, Wu and Hu8) . d-Gal overload induces changes that resemble the biological ageing process and are considerably similar to changes occurring in natural senescence models of neurological impairment, decreased activities of antioxidant enzymes and accelerated tissue ageing(Reference Xu, Xie and Gui5,Reference Haider, Liaquat and Shahzad6) . The d-gal-induced ageing model has been frequently used for brain ageing and anti-ageing pharmacology studies.
Currently, there is interest in the use of natural bioactive products of marine organisms as antioxidants, for example, from marine macroalgae. Porphyra yezoensis is a rich macroalgal resource in Asia that is valued for its nutritional properties, containing abundant polysaccharides (especially sulphated polysaccharides), proteins, phenolic compounds, etc. The macroalgae is used as a vegetable and is believed to have value as a medicine to slow the ageing process(Reference Isaka, Cho and Nakazono9,Reference Takahashi, Hirano and Araki10) . In recent years, sulphated polysaccharides and phycobiliproteins from marine algae have been reported to have powerful antioxidant activities for scavenging the free radicals and preventing oxidative damage in living organisms(Reference Guo, Xu and Zhang11–Reference Zhang, Li and Liu13). In addition, phenolic compounds are other widely studied substances with antioxidant activities(Reference Kähkönen, Hopia and Vuorela14,Reference Rice-Evans, Miller and Paganga15) . However, little effort has been applied to evaluating the possible protective effects of antioxidants against d-gal-induced neurotoxicity and in the treatment of neurodegenerative diseases.
In the present study, we investigated the antioxidative activities of P. yezoensis enzyme degradation extract (PYEDE) in vitro. We also established an animal ageing model by adapting the classic method of rodents chronically injected with d-gal. The objective of the present study was to assess the protective effects of PYEDE on brain injury and neurodegenerative diseases induced by d-gal.
Materials and methods
Chemicals
d-Gal, ascorbic acid (VC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) were supplied by Sinopharm Chemical Reagent Co. Ltd. Assay kits for the measurements of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), reduced glutathione (GSH), malondialdehyde (MDA), total antioxidant capability (T-AOC), nitric oxide (NO), NO synthase (NOS), protein carbonyl (PC), acetylcholinesterase (AchE), monoamine oxidase (MAO), acetylcholine (Ach) and bicinchoninic acid were purchased from Jiancheng Bioengineering Institute. ELISA kits for dopamine (DA) and noradrenaline (NA) were obtained from Sigma-Aldrich. All other chemicals and reagents used in the present study were of analytical grade.
Sample preparation and composition analysis
P. yezoensis was provided by the Lianyungang Youhai Trading Co. Ltd. Agarase was purchased from Sigma Aldrich Pvt. Ltd, and pectinase and cellulase were procured from Heshibi Biotechnology Co. Ltd. After drying in an oven at 50°C for 12 h, the P. yezoensis samples were ground into powder that could be shifted through a size 80 (0·180 mm) mesh sieve by a pulveriser. The samples were degraded by compound enzymes (50 U/g agarase, 90 U/g pectinase and 250 U/g cellulase) for 12 h under the conditions of solid:liquid ratio (water as solvent), hydrolysis temperature and pH of 1:40, 40°C and 6·0, respectively. The mixture was centrifuged at 4800 rpm for 10 min, and the supernatant was collected and freeze-dried to obtain PYEDE. PYEDE was stored at −20°C until further use.
The carbohydrate content in PYEDE was measured by the phenol–sulphuric acid method, and glucose was added as a standard(Reference Nielsen and Nielsen16). The reducing sugar content was assayed using the 3,5-dinitrosalicylic acid method and compared with the standard curve of d-gal(Reference Miller17). The protein content was estimated by the Kjeldahl method(Reference Bradstreet18). The ninhydrin method was used for the quantitative determination of amino acids(Reference Sun, Lin and Weng19). The sulphuric radical content was evaluated by barium sulphate turbidimetry(Reference Sörbo20). The uronic acid content was measured according to the reported method(Reference Blumenkrantz and Asboe-Hansen21). The Folin–Ciocalteu method was used to determine total phenolic compounds(Reference Stratil, Klejdus and Kubáň22).
In vitro determination of antioxidant activity of Porphyra yezoensis enzyme degradation extract
Assay of 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity
The DPPH radical scavenging activity assay was carried out based on the reported procedure with slight modifications(Reference Singh, Kaur and Singh23). Briefly, the sample was prepared in deionised water to obtain various concentrations. Then, 2 ml of a 2 mm methanol solution of DPPH was mixed with 2 ml of the sample. The solutions were fully mixed and incubated at room temperature in the dark for 30 min. After which, the absorbance was measured by a 722S spectrophotometer (Shanghai Precision Instruments Co. Ltd) at 517 nm. VC was used as the positive control, and the DPPH radical scavenging ability was calculated according to the following equation:
where A 0 is the absorbance of the control (methanol instead of the sample), A 1 is the absorbance of the sample and A 2 is the absorbance of the sample under identical conditions as A 1 with methanol instead of DPPH solution.
Assay of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity
The scavenging activity towards ABTS was evaluated according to a published method(Reference Xie, Hu and Wang24). The ABTS radical solution was formed by a 12 h reaction of ABTS (7 mm) with potassium persulphate (K2S2O8, 2·45 mm) at room temperature in the dark. The ABTS solution was diluted with PBS (0·2 m, pH 7·4) to create the working solution with an absorbance of 0·7 (SD 0·02) at 734 nm. Then, 2·4 ml of the ABTS working solution was mixed with 0·6 ml of the sample. After reaction for 6 min at room temperature, the absorbance at 734 nm was measured. VC was used as the positive control, and the ABTS radical scavenging ability was calculated according to the following equation:
where A 0 is the absorbance of the control (water instead of the sample), A 1 is the absorbance of the sample and A 2 is the absorbance of the sample only (PBS instead of ABTS).
Assay of hydroxyl radical scavenging activity
This assay was performed using the published method with slight modifications(Reference Li, Jiang and Zhang25). In brief, 1 ml of FeSO4 (9 mm), 1 ml of 9 mm ethanol solution of salicylic acid and 1 ml of H2O2 (8 mm) were mixed with 1 ml of the sample. After reaction at 37°C for 30 min, the absorbance at 510 nm was measured. VC was used as the positive control, and the hydroxyl radical scavenging ability was calculated according to the following equation:
where A 0 is the absorbance of the control (water instead of the sample), A 1 is the absorbance of the sample and A 2 is the absorbance of the sample only (water instead of H2O2).
Assay of superoxide radical scavenging activity
The ability to scavenge the superoxide radical was determined as previously described by Di et al. (Reference Di, Chen and Sun26) with slight modifications. An aliquot (1 ml) of the sample was mixed with 4·5 ml TRIS-HCl buffer (pH 8·2, 50 mm) containing EDTA (1 mm); then 0·3 ml of pyrogallic acid (3 mm) was added to the mixture and incubated for 5 min at 25°C. Then, we rapidly added 1 ml HCl (8 m) to terminate the reaction and measured the absorbance at 325 nm. VC was used as the positive control, and superoxide anion radical scavenging ability was calculated according to the following equation:
where A 0 is the absorbance of the control (water instead of the sample), A 1 is the absorbance of the sample and A 2 is the absorbance of the sample only (water instead of pyrogallic acid).
In vivo assessment of the effects of Porphyra yezoensis enzyme degradation extract on d-galactose-treated mice
Animals and experimental design
Female ICR mice(Reference Yan, Wang and Wang27,Reference Zhou, Dong and Xu28) (18–20 g) at 3 weeks of age were purchased from Vital River Laboratory Animal Centre (Beijing, China; Licensed ID: SCXK2012-0001). Animals were kept in a polyacrylic cage and maintained under constant conditions (room temperature 23 ± 1°C and humidity 50 ± 10 %) with alternative 12 h light–12 h dark cycles. The mice had free access to rodent food and water.
After 7 d of acclimatisation, all animals were randomly assigned to five groups of twelve animals each: blank control (BC) group, model control (MC) group, positive control group (VC, 90 mg/kg), PYEDE-L group (dose of 50 mg/kg) and PYEDE-H group (dose of 300 of mg/kg). d-Gal was administered at a dose of 400 mg/kg by subcutaneous injection, except for the BC group, which received normal saline (0·9 %, w/w), for 10 weeks. Starting in week 7, the mice in VC and PYEDE groups (distilled water was used to dissolve VC and PYEDE) were given corresponding drug doses by oral gavage once a day for 4 weeks. The mice in the BC and MC groups were given distilled water in the same volumes. All mice experiments were approved by the Ethical Committee of Experimental Animal Care at Ocean University of China (certificate no. SYXK2012014). All animal experiments were conducted in accordance with the line of legislation and ethical guidelines of People’s Republic of China.
Body weight measurement and organ indexes
The mice were weighed every 2 d. On the last day of the experimental period, after 8–12h of fasting, the mice were killed under ether narcotisation, based on the methods described by Tu et al. (Reference Tu, Chang and Chou4). The brain, spleen and thymus were isolated and weighed to calculate the organ coefficients, using the following formula:
Biochemical examinations of malondialdehyde, protein carbonyl and reduced glutathione levels in serum
All the mice were anaesthetised, and 0·5–0·6 ml of peripheral blood was drawn through the angular vein(Reference Zhou, Zhu and Liang29). Blood samples were placed in centrifuge tubes and clotted for 30 min at room temperature and then centrifuged at 5000 rpm for 20 min at 4°C. Finally, the supernatant was separated for biochemical analysis of MDA, PC and GSH levels based on the manufacturer’s instructions for the kits.
Assessment of oxidative status in brain
A random subset of brain samples (nine per group) was collected and homogenised in volumes of ice-cold physiological saline to obtain a 10 % (w/v) homogenate with ten strokes at 4000 rpm in a Bioprep-6 homogenizer (Hangzhou Allsheng Instruments Co. Ltd). According to the preparation of brain homogenates by Xu et al. (Reference Xu, Xie and Gui5), the homogenates were directly centrifuged at 2500 rpm at 4°C for 10 min and the supernatants were obtained to determine MDA, PC and T-AOC levels; SOD, MAO, GSH-PX, NOS and AchE activities; and GSH, NO, Ach, DA and NA contents, according to the corresponding instructions for the kits. Protein concentrations were measured using a commercially available bicinchoninic acid protein assay kit and using bovine serum albumin as a standard.
Determination of neuropathological alterations in the brain
For histological analysis, the brain tissues of three randomly selected mice from different groups were fixed in a fresh solution of 4 % paraformaldehyde (pH 7·4) at 4°C for 24 h, followed by embedment in paraffin and longitudinal sectioning. Then, 5 μm thick sections were obtained for haematoxylin–eosin staining. The stained slides were viewed by microscopy for histopathological analysis.
The method for preparing the brain homogenate was the same as in the ‘Assessment of oxidative status in brain’ section. The supernatants were obtained to determine iNOS activity and NO content based on the manufacturer’s instructions for the kits.
Statistical analysis
The experimental data were analysed by PASW statistics 18 software and subjected to one-way ANOVA, followed by least significant difference (LSD) tests for multiple comparisons. All data are presented as mean values and standard deviations, and values of P < 0·05 and P < 0·01 were considered statistically significant.
Results
The main compositions of Porphyra yezoensis enzyme degradation extract
As shown in Table 1, the major constituents of PYEDE were polysaccharides and proteins. The carbohydrate and protein contents were 222·9 and 211·6 mg/g, respectively. The contents are reducing sugar, sulphuric radical, amino acids, uronic acid and phenolic compounds and were assayed as 132·2, 122·5, 62·3, 19·5, and 6·9 mg/g PYEDE, respectively.
Table 1. The main composition of Porphyra yezoensis enzyme degradation extract (PYEDE)

In vitro antioxidant activity of Porphyra yezoensis enzyme degradation extract
The four widely used assays were performed to measure the antioxidant activity of PYEDE in vitro, and the results are displayed in Fig. 1. The IC50 value was measured as the concentration required to scavenge 50 % of the radicals.

Fig. 1. Scavenging activities on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical (a), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical (b), hydroxyl radical (c) and superoxide radical (d) of Porphyra yezoensis enzyme degradation extract (PYEDE) and ascorbic acid (VC). To determine the antioxidant activity of PYEDE in vitro, VC was used for comparison purposes. Data are presented as mean values and standard deviations of triplicates. ![]() , VC;
, VC; ![]() , PYEDE.
, PYEDE.
The DPPH and ABTS free radicals are widely used indicators of the preliminary radical scavenging capacity of antioxidant compounds(Reference Di, Chen and Sun26). The scavenging capacities for DPPH and ABTS radicals are shown in Fig. 1(a) and (b), respectively. PYEDE and VC showed strong dose-dependent scavenging activity of DPPH and ABTS radicals. The IC50 values for the DPPH and ABTS scavenging activities of VC were 0·013 and 0·008 mg/ml, respectively. The IC50 values of PYEDE corresponded to 2·369 and 2·217 mg/ml, respectively, and the results showed that PYEDE had some DPPH and ABTS radical scavenging abilities.
The superoxide radical is considered as the primary ROS, and the hydroxyl radical is a typical representative of harmful secondary ROS that can cause oxidative injury and cell damage(Reference Xu, Xie and Gui5). As shown in Fig. 1(c) and (d), the antioxidant capacities of PYEDE and VC increased in a concentration-dependent manner. The IC50 values of hydroxyl and superoxide radicals of VC were found to be 0·136 and 0·019 mg/ml, respectively, whereas that of PYEDE were 2·938 and 4·656 mg/ml, respectively. PYEDE exhibited greater hydroxyl and superoxide radicals scavenging capacities compared with VC. As a result, the antioxidant activities of PYEDE were further investigated.
Effect of Porphyra yezoensis enzyme degradation extract on d-galactose-induced ageing model mice
Effect of Porphyra yezoensis enzyme degradation extract on body weight and organ indexes
Morphological changes are inevitable during the ageing process(Reference Chen, Chen and Zhou30). As shown in Fig. 2(a), although the five groups started at the same level, the body weight of the four groups injected with d-gal was similar before the intervention of the test substance, whereas the average body weight of the BC group was markedly higher than those of the other four groups. The experimental animals did not have adverse reactions throughout the experiment. Moreover, after the VC or PYEDE administration, the MC group experienced weight loss, while the VC, PYEDE-L and PYEDE-H groups continued to show increased body weight to varying degrees; the body weights of VC and PYEDE-H groups were similar to that of the BC group. At the end of the experiment, the body weight of the MC group very significantly decreased compared with the BC group (P < 0·05). Compared with the BC group, the MC group showed a similar situation with regard to the organ indexes of the brain, spleen and thymus (P < 0·05 or P < 0·01); the organ indexes decreased significantly after 8 weeks of subcutaneous injection with d-gal (Fig. 2(b)). In contrast, after treatment with VC and PYEDE, the phenomena were substantially alleviated. Overall, the results suggested that to a certain extent, the administration of PYEDE was protected against weight loss and organ atrophy.

Fig. 2. Effect of Porphyra yezoensis enzyme degradation extract (PYEDE) on body weight (a) and organ indexes (b) on d-galactose-induced ageing mice. Data are given as mean values and standard deviations (n 12). † P < 0·05 and †† P < 0·01 v. blank control (BC) group. * P < 0·05 and ** P < 0·01 v. model control (MC) group. (a) ![]() , BC;
, BC; ![]() , MC;
, MC; ![]() , ascorbic acid (VC);
, ascorbic acid (VC); ![]() , low-dose PYEDE (PYEDE-L);
, low-dose PYEDE (PYEDE-L); ![]() , high-dose PYEDE (PYEDE-H). (b)
, high-dose PYEDE (PYEDE-H). (b) ![]() , BC;
, BC; ![]() , MC;
, MC; ![]() , VC;
, VC; ![]() , PYEDE-L;
, PYEDE-L; ![]() , PYEDE-H.
, PYEDE-H.
Effect of Porphyra yezoensis enzyme degradation extract on malondialdehyde, protein carbonyl and reduced glutathione levels in serum
The degree of lipid peroxidation and protein oxidative damage are conventionally assessed by the level of MDA and the quantification of carbonyl groups, and GSH plays a significant role in the intracellular antioxidant defence of the body(Reference Çoban, Doğan-Ekici and Aydın31,Reference Mohammadi, Mehri and Bostan32) . Therefore, we evaluated the oxidative damage in the body by measuring the biochemical indicators MDA, PC and GSH. As illustrated in Fig. 3(a) and (b), the levels of MDA and PC noticeably increased (by 11·9 and 55·2 %, respectively) in the serum of the model mice compared with those in the BC group (P < 0·05 or P < 0·01). In contrast, the content of GSH was 23·3 % lower compared with the normal mice (Fig. 3(c)). VC administration at 90 mg/kg substantially decreased the MDA and PC levels (by 17·1 and 40·0 %, respectively) v. those in the MC group (both P < 0·01), but there were no statistically significant differences in the GSH content (P > 0·05). Supplementation with 300 mg/kg PYEDE alleviated the effect, resulting in an evident decrease in MDA and PC levels and an increase in GSH content in the serum (P < 0·05 or 0·01). These findings suggested that PYEDE attenuated d-gal-induced lipid peroxidation and protein carbonylation and improved the ability of the mice to defend against antioxidants.

Fig. 3. Effects of Porphyra yezoensis enzyme degradation extract (PYEDE) treatment on the levels of malondialdehyde (MDA) (a), protein carbonyl (PC) (b) and reduced glutathione (GSH) (c) content in serum. Data are given as mean values and standard deviations (n 12). † P < 0·05 and †† P < 0·01 v. blank control (BC) group. * P < 0·05 and ** P < 0·01 v. model control (MC) group. VC, ascorbic acid; PYEDE-L, low-dose PYEDE; PYEDE-H, high-dose PYEDE.
Effect of oxidative status in the brain
Numerous studies have proved that antioxidant activity plays an indispensable role in the biological ageing process(Reference Rehman, Shah and Ali3). Thus, we further investigate several key antioxidants that can scavenge ROS, including the enzymatic antioxidants GSH-Px and SOD and the non-enzymatic antioxidant T-AOC in the brain. The differences in the brain antioxidant parameters between the treatment groups are shown in Table 2. The d-gal group showed remarkably decreased GSH-Px (by 32·6 %), SOD (by 17·2 %) and T-AOC (by 51·9 %) activities (all P < 0·01) relative to the BC group. Moreover, the challenge by d-gal led to a significant decrease in GSH (by 28·9 %, P < 0·05) but an increase in MDA and PC contents by 1·26-fold and 1·44-fold, respectively (P < 0·05 or 0·01). When PYEDE was administered at a low dose (50 mg/kg), the activities of SOD and T-AOC and the level of GSH were 13·3, 40·4 and 33·3 % (P < 0·05 or 0·01) higher compared with the MC group, respectively. Meanwhile, the contents of MDA and PC clearly decreased by 16·6 and 24·5 % (P < 0·05 or 0·01), respectively, but the variation in the activity of GSH-Px in the brain did not reach statistical significance (P > 0·05). In addition, significantly higher activities of GSH-Px, SOD and T-AOC and a higher level of GSH were found in PYEDE-H (300 mg/kg) and VC groups, and a significant decrease in the contents of MDA and PC occurred (P < 0·05 or 0·01). PYEDE (300 mg/kg) exhibited similar effects to VC, and PYEDE was better for improving the levels of T-AOC and GSH. These results collectively indicated that PYEDE protected against oxidative stress damage induced by d-gal.
Table 2. Effects of Porphyra yezoensis enzyme degradation extract (PYEDE) treatment on glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capability (T-AOC) activities and malondialdehyde (MDA), protein carbonyl (PC) and reduced glutathione (GSH) contents in the brain of d-galactose-induced mice(Mean values and standard deviations)

BC, blank control; MC, model control; VC, ascorbic acid; PYEDE-L, low-dose PYEDE; PYEDE-H, high-dose PYEDE.
* P < 0·05 and ** P < 0·01 v. MC.
† P < 0·05 and †† P < 0·01 v. BC.
Analysis of Porphyra yezoensis enzyme degradation extract on brain neuropathological alterations
The CA1 region of the hippocampus is known to be vital for learning and memory, and the neurogenesis in the hippocampus declines with ageing, along with a continuous loss of neurons(Reference Deupree, Bradley and Turner33). As depicted in Fig. 4(a), no pathological alterations were evident in the BC group, the nerve fibres and neurons were arranged regularly and tightly and the nuclei were large, round and lightly stained in the CA1 region of the hippocampus. In contrast, there were some neuropathological changes in the MC group, including a reduction of the CA1 pyramidal cell layer, irregular nerve fibres and loosely arranged neurons, accompanied by atrophy or disappearance. After administration of VC and PYEDE, the morphology of the nerve fibres and neurons visibly improved compared with the MC group; there was an obvious increase in round-shaped neurons with regular nerve fibres, and neurons were more compactly and orderly arranged. The effect of PYEDE at high dose (300 mg/kg) appeared similar to those in the BC and VC groups. In addition, the iNOS activity and NO content, which lead to neurodegenerative diseases, markedly increased (P < 0·01) in d-gal-induced mice compared with normal mice (Fig. 4(b)). After administration of PYEDE and VC, the iNOS activity and NO content significantly down-regulated compared with those in the MC group. The results suggested that PYEDE attenuated neurodegenerative diseases caused by oxidative stress and improved cognitive ability.

Fig. 4. The neurodegenerative changes in the CA1 region of the hippocampus. (a) Photomicrographs of haematoxylin–eosin (H&E) staining in the CA1 region of the hippocampus (H&E staining, magnification 200×) (n 3). Apoptosis and irregular neurons are marked by the black and orange arrows, respectively. (b) Inducible nitric oxide synthase (iNOS) activity and nitric oxide (NO) content in the hippocampus. Data are given as mean values and standard deviations (n 9). †† P < 0·01 v. blank control (BC) group. * P < 0·05 and ** P < 0·01 v. model control (MC) group. VC, ascorbic acid; PYEDE-L, low-dose PYEDE; PYEDE-H, high-dose PYEDE.
Neuroprotective effect of Porphyra yezoensis enzyme degradation extract on d-galactose-induced ageing of the mice brain
The symptoms of neurodegenerative diseases include memory impairment, the loss of cognitive ability and dyskinesia. It is well documented that these symptoms are also associated with the disorders of the metabolism of cholinergic and monoamine neurotransmitters(Reference Kou, Zhu and Yan2,Reference Xia, Xing and Li34) . Therefore, we studied the effect of PYEDE on the AchE activity and Ach content of the cholinergic nervous system and the MAO activity and DA and NA contents of monoamine neurotransmitters. Fig. 5 reveals the data on neuroprotection in the mice brain. Long-term d-gal injection in mice caused a 1·20-fold increase in AchE enzymatic activity (P < 0·01) (Fig. 5(a)) but a significant decrease in the Ach content (by 16·2 %, P < 0·05) (Fig. 5(b)) of the brain compared with those of the BC group. Similarly, MAO activity dramatically increased 1·43-fold (P < 0·01) (Fig. 5(c)), and DA and NA contents were 13·4 and 10·4 % (both P < 0·01) (Figs. 5 (d) and (e)) lower, respectively, v. the normal mice. While VC obviously inhibited the increase in AchE and MAO activities (P < 0·01) and Ach and NA contents were remarkably enhanced 1·29-fold and 1·11-fold (both P < 0·01), respectively, there was no significant improvement in DA content (P > 0·05). In addition, PYEDE remarkably and dose-dependently exhibited neuroprotective effects, and the protective effect of 300 mg/kg PYEDE visibly exceeded that of VC. Our findings revealed that PYEDE markedly alleviated nervous system damage induced by d-gal.

Fig. 5. Effect of Porphyra yezoensis enzyme degradation extract (PYEDE) on activities of acetylcholinesterase (AchE) (a), monoamine oxidase (MAO) (c) and contents of acetylcholine (Ach) (b), dopamine (DA) (d), noradrenaline (NA) (e) in the brain of d-galactose-induced mice. Data are given as mean values and standard deviations (n 9). † P < 0·05 and †† P < 0·01 v. blank control (BC) group. * P < 0·05 and ** P < 0·01 v. model control (MC) group. VC, ascorbic acid; PYEDE-L, low-dose PYEDE; PYEDE-H, high-dose PYEDE.
Discussion
Ageing is a major cause of physiological dysfunctions, including central nervous system degeneration, cognitive deterioration and the massive loss of neurons. With the increasing population and prolongation of lifespans, ageing has become a worldwide problem and anti-ageing has become an important public issue(Reference Rehman, Shah and Ali3,Reference Chen, Chen and Zhou30) . The brain accounts for 20 % of the total VO2 of the body; it is prone to a lack of antioxidant enzymes and produces relatively more ROS compared with other tissues with low VO2(Reference Gusnard and Raichle35). There is considerable evidence that oxidative stress plays an important role during the pathogenesis of age-associated or neurodegenerative diseases. An imbalance between the generation of oxidants and the antioxidant defences of the body leads to oxidative damage of cells and tissues, modifying their morphology and function and resulting in ageing and premature cell death(Reference Tu, Chang and Chou4,Reference Xu, Xie and Gui5) . The d-gal-induced ageing model is based on the metabolic and free radical theories of ageing and is similar to the natural ageing process(Reference Hsieh, Wu and Hu8,Reference Çoban, Doğan-Ekici and Aydın31) . Therefore, the chronic d-gal-induced ageing of mice has been widely used in anti-ageing pharmacology studies. This model was used to investigate supplements and antioxidants that have the potential to treat brain damage caused by ageing.
P. yezoensis is a macroalga with high nutritive value that is mainly distributed in Asia; numerous studies have reported that the cell wall of red algae is mainly composed of an abundance of polysaccharides, such as agar, cellulose and pectin(Reference Takahashi, Hirano and Araki10). In the present study, we used agarase, cellulase and pectinase to degrade the cell wall of P. yezoensis, as, compared with other methods, the intracellular components can be released more effectively, resulting in increased dissolution of active and nutritional ingredients. We used four classic established in vitro antioxidant systems to evaluate the antioxidant properties of PYEDE. Our study found that PYEDE had significant scavenging abilities on DPPH, ABTS, hydroxyl and superoxide radicals, proving that PYEDE has antioxidant potential. This activity can be attributed to the composition of PYEDE. Previous studies have explored the ability of P. yezoensis polysaccharides to prevent acute chemical liver injury induced by CCl4 in mice(Reference Guo, Xu and Zhang11). Previous investigations have verified that C-phycoerythrin is involved in the amelioration of diabetic complications by significant reductions in oxidative stress(Reference Soni, Visavadiya and Madamwar12). In addition, some phenolic compounds have strong antioxidant properties(Reference Singh, Kaur and Singh23).
To further confirm the potential of PYEDE as an antioxidant, we established an ageing model by subcutaneous injections of d-gal into female ICR mice for 10 weeks to investigate the protective effect of PYEDE on brain injury and neurodegenerative diseases induced by d-gal. Previous studies have shown that the most easily assessed features of the ageing process are the morphological changes in appearance, and d-gal can accelerate these features(Reference James, Michalek and Pitiyage36). In our study, before the intervention treatment, the body weight of normal mice was higher than the d-gal-treated mice and we were able to effectively alleviate weight loss after PYEDE intervention treatment. The d-gal-induced ageing model group showed slow weight loss, which was similar to the results of previous studies(Reference Xia, Xing and Li34,Reference Gong, Guo and Hu37) . The brain is an important part of the central nervous system and the main regulator of vital functions. In addition, the spleen and thymus are considered to be indispensable immune organs, and spleen and thymus indexes can reflect the immune function of the body and are precise indicators of biological ageing(Reference Zhang, Wu and Lin38). There were significant decreases in body weight and organ indexes of brain, spleen and thymus indexes after administration of d-gal, and similar consequences have been found in previous studies(Reference Chen, Chen and Zhou30,Reference Fatemi, Khaluoi and Kaeidi39) . In our study, PYEDE effectively alleviated weight loss and organ atrophy, suggesting that PYEDE protected the brain from atrophy and slowed ageing by strengthening immune functions.
Oxidative stress caused by ROS is the major driving force of brain damage, as it may directly oxidise lipids and proteins and indirectly induce oxidant/antioxidant imbalances by disrupting the activity of enzymes(Reference Chen, Chen and Zhou30). It is well documented that d-gal-induced oxidative damage can be evaluated by measuring the activities of antioxidant enzymes (GSH-Px, SOD, MAO and T-AOC) and the levels of MDA, PC and GSH(Reference Rehman, Shah and Ali3,Reference Hsieh, Wu and Hu8,Reference Lu, Zheng and Wu40) . As one of the most important members of the antioxidant defence system, SOD can catalyse the superoxide radical to form H2O2, which can be further metabolised by GSH-Px. GSH is a significant antioxidant and free radical scavenger in the body; it can react with H2O2 to form GSSH under the catalysis of GSH-Px and remove peroxide and hydroxyl radicals produced by cellular respiratory metabolism(Reference Mohammadi, Mehri and Bostan32,Reference Peng, Kong and Yu41) . T-AOC has comprehensive antioxidant capacities, whereas MDA is a by-product of lipid peroxidation induced by free radicals, and its content indirectly reflects the level of lipid peroxidation in the body. The generation of PC is an important marker of the oxidative modification of protein molecules by free radicals. The level of PC is closely related to ageing and is often used to indicate the extent of oxidative damage in proteins(Reference Çoban, Doğan-Ekici and Aydın31,Reference Aydın, Çoban and Doğan-Ekici42) . In the present study, after treatment with PYEDE, MDA and PC levels were clearly reduced and the content of GSH remarkably elevated in serum. Serum is the most intuitive indicator of physical status, suggesting that PYEDE could protect the mice against d-gal-induced oxidative stress. Oxidative damages are a crucial factor which contributing to brain ageing and senile dementia(Reference Kou, Zhu and Yan2). Furthermore, we observed that administration of PYEDE improved the antioxidant activities of GSH-Px, SOD and T-AOC and the level of GSH, as well as reduced MDA and PC levels in the brain. These results were similar to those of a study on carnosine and taurine(Reference Aydın, Çoban and Doğan-Ekici42) in which PYEDE substantially inhibited the occurrence of oxidative damage in the brain. In addition, the protective antioxidant effects of a P. yezoensis polysaccharide in CCl4 hepatotoxicity have been reported(Reference Guo, Xu and Zhang11). These data provide evidence that the bioactivities of P. yezoensis could be used to prevent oxidative stress.
NO is considered as an important diffusion signal in brain development, learning and memory, but excessive production of NO is cytotoxic, causing nerve damage and encephalopathy(Reference Suzuki, Motohashi and Uezumi43). NO is synthesised by at least three isozymes (inducible NOS, neuronal NOS and endothelial NOS). In contrast to neuronal NOS and endothelial NOS, which are known as constitutive NOS, iNOS is not expressed in the brain under normal physiological conditions and has been proven to be an inhibitory molecule of neuron regeneration in the hippocampus(Reference Necchi, Virgili and Monti44,Reference Thorns, Hansen and Masliah45) . Our study demonstrated that iNOS activity and NO content were up-regulated in d-gal-induced mice. In addition, histopathological analysis revealed a reduction of the pyramidal cell layer and severe damage to neurons, accompanied by irregular nerve fibres in the hippocampus CA1 region in d-gal-treated mice brain, and the findings were consistent with previous studies(Reference Xu, Xie and Gui5,Reference Zhang, Wu and Lin38) . PYEDE treatment can effectively inhibit the activity of iNOS, reduce the content of NO and alleviate damage to the hippocampus and brain atrophy, possibly by renewing the ability of the brain to scavenge ROS (in vitro) and reducing lipid peroxidation and protein carbonylation. Previous studies have shown that inhibition of iNOS and NO generation can attenuate hippocampal neuronal apoptosis, and an ethanol extract of P. yezoensis promoted the development of hippocampal neurons by enhancing the rates of early neuronal differentiation and axodendritic arborisation(Reference Mohibbullah, Bhuiyan and Hannan46,Reference Yuan, Guo and Deng47) . Another study demonstrated that d-gal affected inflammatory markers, such as iNOS, causing them to activate the NF-κB pathway(Reference Rehman, Shah and Ali3). At present, the pathogenesis and aetiology of many neurodegenerative diseases are not fully understood; however, many researchers believe that a reduction of neurons is one of the main pathogeneses, and this mechanism is targeted in the search for improved methods and drugs.
The metabolism of neurotransmitters plays a crucial role in the regulation of the neuroendocrine network, and alterations in multiple neurotransmitter systems may be associated with the pathogenesis of age-related brain degenerative disorders such as Alzheimer’s disease(Reference Kou, Zhu and Yan2,Reference Ng, Papandreou and Heales48) . The complex aetiology of Alzheimer’s disease has encouraged active research into developing multi-target drugs with two or more complementary biological activities. The development of novel inhibitors of AchE and MAO is a promising direction for the treatment of Alzheimer’s disease(Reference Viña, Matos and Yáñez49). AchE and MAO are enzymes relevant to cholinergic and monoaminergic neurotransmitters, respectively. AchE is an important regulatory enzyme that controls the transmission of nerve impulses across cholinergic synapses by hydrolysing Ach to choline and acetate(Reference Kaizer, da Silva and Morsch50). Increased activity of AchE is associated with the formation of amyloid plaques in the brain, and increased levels of β-amyloid can, eventually, cause degeneration of cholinergic nerve terminal function in the hippocampus and cholinergic neuron atrophy, causing disruption of neurotransmission and eventually triggering Alzheimer’s disease(Reference Chauhan and Siegel51–Reference Lim, Maruff and Schindler53). MAO is an important enzyme of the outer mitochondrial membrane and the central nervous system that catalyses the degradation of a wide range of monoamine neurotransmitters, including DA and NA(Reference Çoban, Doğan-Ekici and Aydın31,Reference Gong, Guo and Hu37) . It is assumed that activation of MAO is associated with the generation of free radicals in the involution of the nervous tissue(Reference Tian, Zou and Yang54). In the present study, increased AchE and MAO activities led to a reduction of Ach and monoamine neurotransmitters in the brains of d-gal-induced mice, whereas PYEDE reduced the activities of AchE and MAO and increased the contents of Ach, DA and NA. Thus, the neuroprotective effects of PYEDE involved modulation of the activities of enzymes related to neurotransmitter metabolism. Numerous studies have also shown that improvements in learning and memory of aged mice were achieved by increasing NA and DA levels and decreasing the activity of AchE(Reference Zhang, Han and Zhang55–Reference Schmatz, Mazzanti and Spanevello57). The polysaccharides of PYEDE might have played a role in the neuroprotective effects, and this is supported by previous studies(Reference Gao, Li and Yin58) that found that oligosaccharides or sulphated oligosaccharides extracted from seaweed, such as GV-971, captured β-amyloid at multiple sites, and inhibited β-amyloid fibrils formation, and improved cognitive impairment. Moreover, oxidative damage induced by free radicals was an important factor in neuronal degeneration and promoted the appearance of β-amyloid and neurofibrillary changes(Reference Peng, Kong and Yu41). Thus, the neuroprotective effects of PYEDE were also achieved by reversing the decline of the antioxidant defence, which was confirmed by the data above. However, the imbalance in neurotransmitters which are synthesised within the neurons led to synaptic damage and neuronal cell loss relevant to memory function(Reference Xu, Rong and Xie56), which is similar to the phenomenon observed in our study. Therefore, it seems that PYEDE ameliorated the cell components of the oxidative damaged brain and restored neuronal activity, including the synthesis and transport of neurotransmitters and enzymes, which is consistent with hippocampal pathology.
Conclusions
In summary, the results of our study support the hypothesis that treatment with PYEDE could alleviate oxidative-stress-induced damage in neurodegenerative diseases. Our findings demonstrated that PYEDE exerted a strong antioxidant effect in vitro and effectively protected against brain injury in d-gal-treated mice. The underlying protective mechanisms might involve the improvement in organ atrophy, inhibition of lipid peroxidation and protein carbonylation, renewal of antioxidant enzymes activities, amelioration of hippocampal neuronal apoptosis and damage and beneficial modulation of multiple neurotransmitter systems. The present study provides novel insights into PYEDE as an effective mediator of age-related cognitive deficits and supports the use of a multitarget approach in the treatment of neurodegenerative diseases.
Ethical statement
All animal treatments were strictly in accordance with the Ethical Committee of Experimental Animal Care at Ocean University of China (certificate no. SYXK2012014). All animal experiments were conducted in accordance with the line of legislation and ethical guidelines of People’s Republic of China.
Acknowledgements
The authors are grateful to Professor Jingfeng Wang for providing experimental sites and experimental technical guidance for animal feeding.
This work was funded by Qingdao Marine biological medicine science and technology innovation center construction project (2017-CXZX01-4-6).
Designed the experiments: C. W., Z. S. and X. J. Prepared the extracts: C. W., J. Y. and F. M. Performed the experiments: C. W., Z. S., J. Y. and F. M. Drafted and revised the manuscript: C. W., Z. S. and C. Z. Funding acquisition and project administration: X. J., C. Z. and Z. S.
The authors declare that they have no conflicts of interest. All authors confirmed the manuscript authorship and agreed to submit it for peer review.