Electroconvulsive therapy (ECT) is one of the most effective and rapid antidepressant treatments for severe depression. Several meta-analyses have reported the superiority of ECT compared with pharmacotherapy for depression.Reference Carney, Cowen, Geddes, Goodwin, Rogers and Dearness1, Reference Pagnin, de Queiroz, Pini and Cassano2 In addition, ECT is more effective for patients with severe depressive symptoms resistant to multiple antidepressant medications.Reference Kellner, Kaicher, Banerjee, Knapp, Shapiro and Briggs3 Clinically important aspects of ECT include more rapid alleviation of depressive symptoms and reduction in suicidality in comparison with pharmacotherapy.Reference Husain, Rush, Fink, Knapp, Petrides and Rummans4–Reference Kellner, Fink, Knapp, Petrides, Husain and Rummans6 Despite the robust clinical efficacy of ECT its mechanisms of action remain elusive. Since the therapy was introduced to clinical practice in the 1930s a wealth of studies have provided several hypotheses, including monoamine neurotransmitter, neuroendocrine and anticonvulsant theories.Reference Kellner, Greenberg, Murrough, Bryson, Briggs and Pasculli7 In addition to these hypotheses, recent studies have focused on ECT-related neuroplastic effects, especially those occurring in the hippocampus. These effects have been derived from animal models. Particularly, a preclinical study showed that electroconvulsive shock (ECS), an animal model of ECT, induced neurogenesis in the dentate gyrus of the hippocampus.Reference Madsen, Treschow, Bengzon, Bolwig, Lindvall and Tingstrom8 This finding has been replicated in several studies.Reference Malberg, Eisch, Nestler and Duman9, Reference Perera, Coplan, Lisanby, Lipira, Arif and Carpio10, Reference Warner-Schmidt and Duman11 Recently Nordanskog et al reported for the first time an ECT-related increase in hippocampal volume in depressed patients.Reference Nordanskog, Dahlstrand, Larsson, Larsson, Knutsson and Johanson12 Furthermore, several magnetic resonance imaging (MRI) studies investigating the effects of ECT on brain structures have been published. However, most of the longitudinal ECT studies had small sample sizes, because patients who need ECT are often severely ill, in need of urgent treatment and have difficulty participating in such studies.Reference Abbott, Gallegos, Rediske, Lemke and Quinn13 Therefore, it is necessary to conduct systematic review and meta-analysis for ECT-related effects on brain structures. Currently there are several narrative or systematic reviews of neuroimaging studies related to ECT,Reference Abbott, Gallegos, Rediske, Lemke and Quinn13–Reference Yrondi, Peran, Sauvaget, Schmitt and Arbus16 but only one meta-analysis has been published so far, that by Wilkinson et al who reported that ECT increased bilateral hippocampal volume.Reference Wilkinson, Sanacora and Bloch17 Unfortunately, they did not include the largest study currently available,Reference Bouckaert, Dols, Emsell, De Winter, Vansteelandt and Claes18 and focused only on hippocampal volume. Our aim was to systematically review the MRI studies investigating structural changes due to ECT in patients with depression (major depression or bipolar affective disorder) and quantitatively analyse whether ECT induces hippocampal and other brain region structural changes, through a meta-analytic approach. An additional aim was to determine the effects of potential moderators, including clinical and demographic factors (e.g. age) and method of ECT (e.g. electrode placement), on the ECT-induced neuroplastic effects.
Method
We followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie19 The literature search, decisions on inclusion, data extraction and quality control were performed independently by two of the authors (A.T. and J.C.). Disagreements about study selection were solved by consensus; when this was not possible another author (T.K.) was contacted to resolve the disagreements. The protocol was not preregistered in any database.
Study selection
We searched Medline, EMBASE and PsycINFO (last search 2 July 2017) to identify eligible studies on changes in structural plasticity of hippocampi and other brain regions with ECT. The following keywords were used: (electroconvulsive OR ECT) AND ('magnetic resonance' OR MRI OR volume). In addition, the reference lists of the included articles were reviewed. The inclusion criteria were that studies were published in English, involved clinically depressed patients (with either major depressive disorder or bipolar affective disorder as defined by DSM or ICD criteria), had a longitudinal design with at least two scans (before and after ECT) and evaluated the effects of ECT on brain structures, including at least hippocampi, using MRI.
Data extraction
Changes in left and right hippocampal volume with ECT were designated as primary outcomes, and the secondary outcomes were changes in left and right amygdala volume with ECT. We extracted the means and standard deviations of hippocampal volumes as well as amygdala volumes. We also extracted clinical characteristics of the samples (number of participants, diagnosis, age, gender, medication status, clinical assessment, comparisons), ECT parameters (machine, electrode placement, treatment frequency, anaesthesia), MRI parameters (magnetic field strength, echo time, repetition time) and time from the last ECT to post-ECT assessment. When actual hippocampal volumes were not reported we extracted t or P values. If the same sample or partially overlapping samples were included in more than one report, data from the study with the largest number of participants were included. Where records did not provide sufficient information, corresponding authors were contacted and the required data were requested. If the authors did not respond or provide information that was essential for the meta-analysis, we excluded the study.
Quality assessment
All articles meeting the eligibility criteria were assessed for their quality by two investigators (A.T. and T.K.) using a modified version of the Newcastle–Ottawa Scale.Reference Wells, Shea, O'Connell, Peterson, Welch and Losos20 This is a quality assessment scale designed to evaluate and validate the quality of non-randomised studies. Our version of the scale was modified to assess longitudinal studies and to produce a comprehensive score, ranging from 0 (lowest quality) to 17 (highest quality), to be assigned to each included study. Total scores were determined by consensus between the two investigators.
Statistical analysis
The analyses were performed using Comprehensive Meta-Analysis version 3.0. The standardised mean difference (SMD) between pre- and post-ECT hippocampal volumes was calculated with 95% confidence intervals, using random effects models. Heterogeneity was reported using τ2, I 2, Q and P values. To assess the robustness of the results, and to identify potential methodological biases and subpopulations in which outcomes differed, we also conducted a series of a priori defined subgroup/sensitivity and meta-regression analyses. In addition to meta-analysing hippocampal volume change in SMD across studies, we expressed the results in terms of raw volume change from studies that reported actual volume changes. We also conducted subgroup analysis and meta-regression analyses to identify potential treatment effect moderators such as medication status (medicated or unmedicated), age (mean age), gender (proportion of female participants), diagnosis (proportion with bipolar affective disorder), electrode placement (proportion of right unilateral placement) and clinical improvement (percentage reduction in depression severity score, percentage of those responding and percentage of those with remitted disorder). For electrode placement (bilateral or right unilateral) we explored the regional effect of direct electrical stimulation, i.e. whether right unilateral (RUL) electrical stimulation would lead to larger effects on right brain volume compared with effects on left brain volume. For medication status we categorised studies into ‘medicated’ or ‘unmedicated’. If a study included both medicated and unmedicated patients, we labelled the study as ‘medicated’. Egger's regression test followed by Duval & Tweedie's trim and fill method were used to assess publication bias.Reference Egger, Davey Smith, Schneider and Minder21, Reference Duval and Tweedie22
Results
We identified 1331 research articles after duplicates were removed. Of these, 1253 studies were excluded at title and abstract level and 78 studies were selected as potentially relevant after initial evaluation. Full details of the search results, including the reasons for exclusion, are summarised in online Fig. DS1 available at https://doi.org/10.1192/bjp.2017.11. A total of 18 studies met the inclusion criteria for our systematic-review (online Table DS1).Reference Nordanskog, Dahlstrand, Larsson, Larsson, Knutsson and Johanson12, Reference Bouckaert, Dols, Emsell, De Winter, Vansteelandt and Claes18, Reference Coffey, Weiner, Djang, Figiel, Soady and Patterson23–Reference Cano, Martinez-Zalacain, Bernabeu-Sanz, Contreras-Rodriguez, Hernandez-Ribas and Via38 A total of 193 participants from eight independent studies were selected for the meta-analysis. Of these, five independent studies (n = 100) reported volume changes in amygdala. Details of the included studies for meta-analysis are presented in Table 1. The results of quality assessments for included studies using a modified Newcastle–Ottawa Scale are presented in online Table DS2.
Table 1 Overview of studies included in the meta-analysis
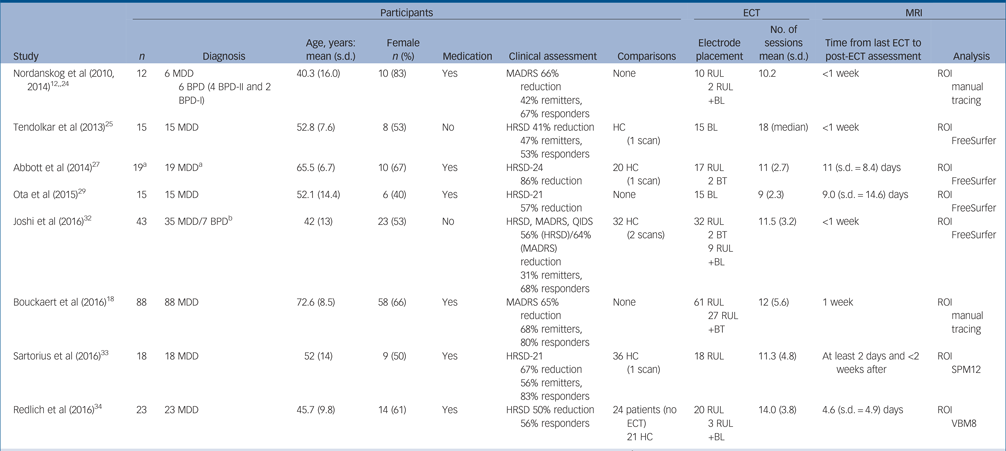
BL, bilateral; BPD, bipolar disorder; BT, bitemporal; ECT, electroconvulsive therapy; HC, healthy control; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery–Åsberg Depression Rating Scale; MDD, major depressive disorder; MRI, magnetic resonance imaging; QIDS, Quick Inventory of Depressive Symptomatology; ROI, region of interest; RUL, right unilateral; SPM, statistical parametric mapping; VBM, voxel-based morphometry.
a. Authors analysed only data from 15 responders.
b. Reported in the original article.
Primary outcomes: hippocampal volume
There were significant hippocampal volume increases both on the right side (8 studies, n = 193, SMD = 0.51, 95% CI 0.27 to 0.76, P < 0.001; heterogeneity: τ2 = 0.10, I 2 = 94%, Q = 122, P < 0.001) and on the left side (8 studies, n = 193, SMD = 0.381, 95% CI 0.18 to 0.58, P < 0.001; heterogeneity: τ2 = 0.07, I 2 = 92%, Q = 86, P < 0.001) with ECT (Fig. 1). Sensitivity analyses of six studies (n = 152) reporting actual hippocampal volume also showed that the right hippocampus (SMD = 0.41, 95% CI 0.15 to 0.68, P = 0.002; heterogeneity: τ2 = 0.10, I 2 = 96%, Q = 111, P < 0.001; differences in means = 200 mm3, 95% CI 87 to 312, P < 0.001; heterogeneity: τ2 = 17837, I 2 = 94%, Q = 87, P < 0.001) and the left hippocampus (SMD = 0.30, 95% CI 0.09 to 0.51, P = 0.005; heterogeneity: τ2 = 0.06, I 2 = 93%, Q = 75, P < 0.001; differences in means = 162 mm3, 95% CI 51 to 273, P = 0.004; heterogeneity: τ2 = 17270, I 2 = 93%, Q = 69, P < 0.001) increased after ECT (online Fig. DS2).
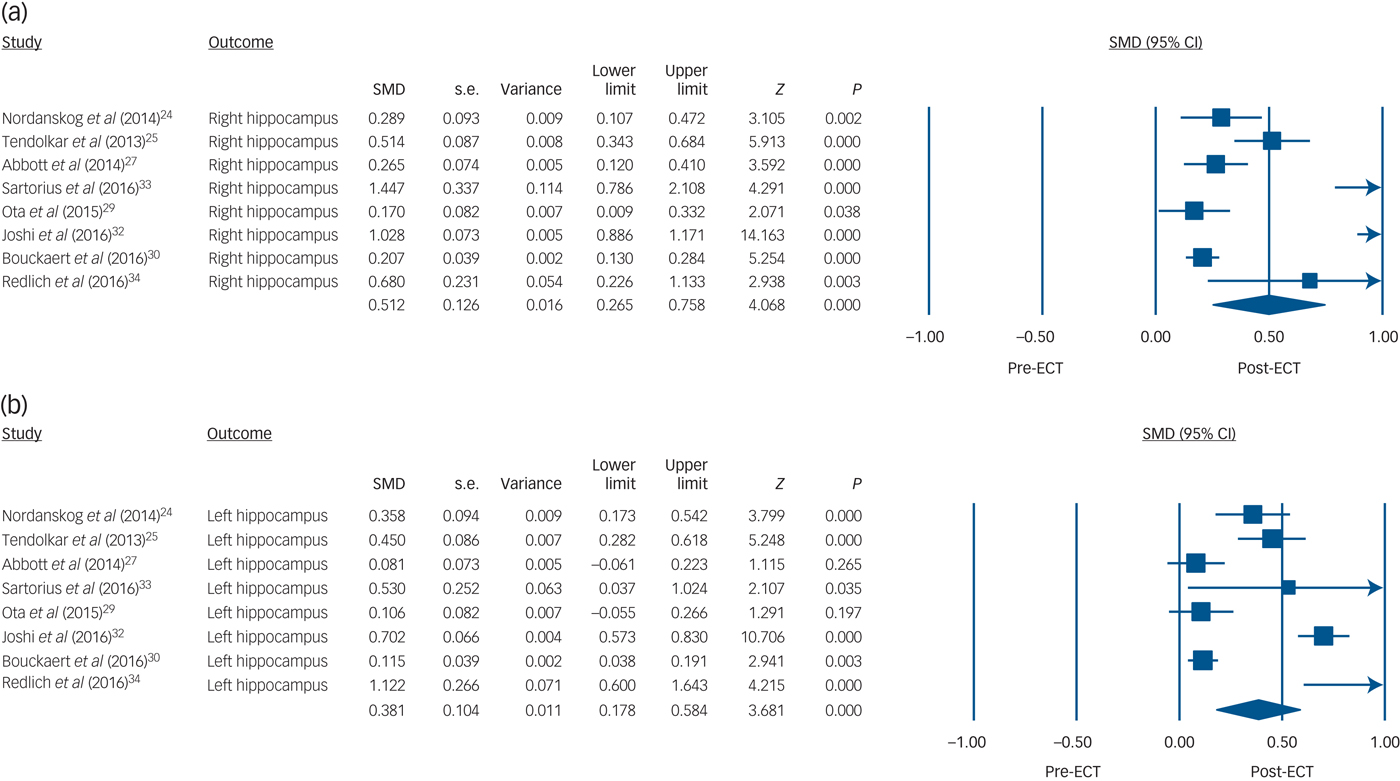
Fig. 1 Volume changes with electroconvulsive therapy (ECT) in (a) the right hippocampus and (b) the left hippocampus.
Secondary outcomes: amygdala volume
There were significant amygdala volume increases both on the right side (5 studies, n = 100, SMD = 0.51, 95% CI 0.32 to 0.70, P < 0.001; heterogeneity: τ2 = 0.03, I 2 = 73%, Q = 15, P = 0.006) and the left side (5 studies, n = 100, SMD = 0.54, 95% CI 0.28 to 0.80, P < 0.001; heterogeneity: τ2 = 0.06, I 2 = 86%, Q = 28, P < 0.001) with ECT (Fig. 2). Sensitivity analyses of three studies (n = 59) reporting actual amygdala volume also showed that the right amygdala (SMD = 0.44, 95% CI 0.25 to 0.62, P < 0.001; heterogeneity: τ2 = 0.02, I 2 = 78%, Q = 8.9, P = 0.012; differences in means = 110 mm3, 95% CI 66 to 156, P < 0.001; heterogeneity: τ2 = 1220, I 2 = 78%, Q = 9.1, P = 0.011) and the left amygdala (SMD = 0.38, 95% CI 0.12 to 0.65, P = 0.005; heterogeneity: τ2 = 0.05, I 2 = 90%, Q = 19, P < 0.001; differences in means = 124 mm3, 95% CI 101 to 147, P < 0.001; heterogeneity: τ2 = 39, I 2 = 6.8%, Q = 2.2, P = 0.34) volumes both increased after ECT (online Fig. DS3).
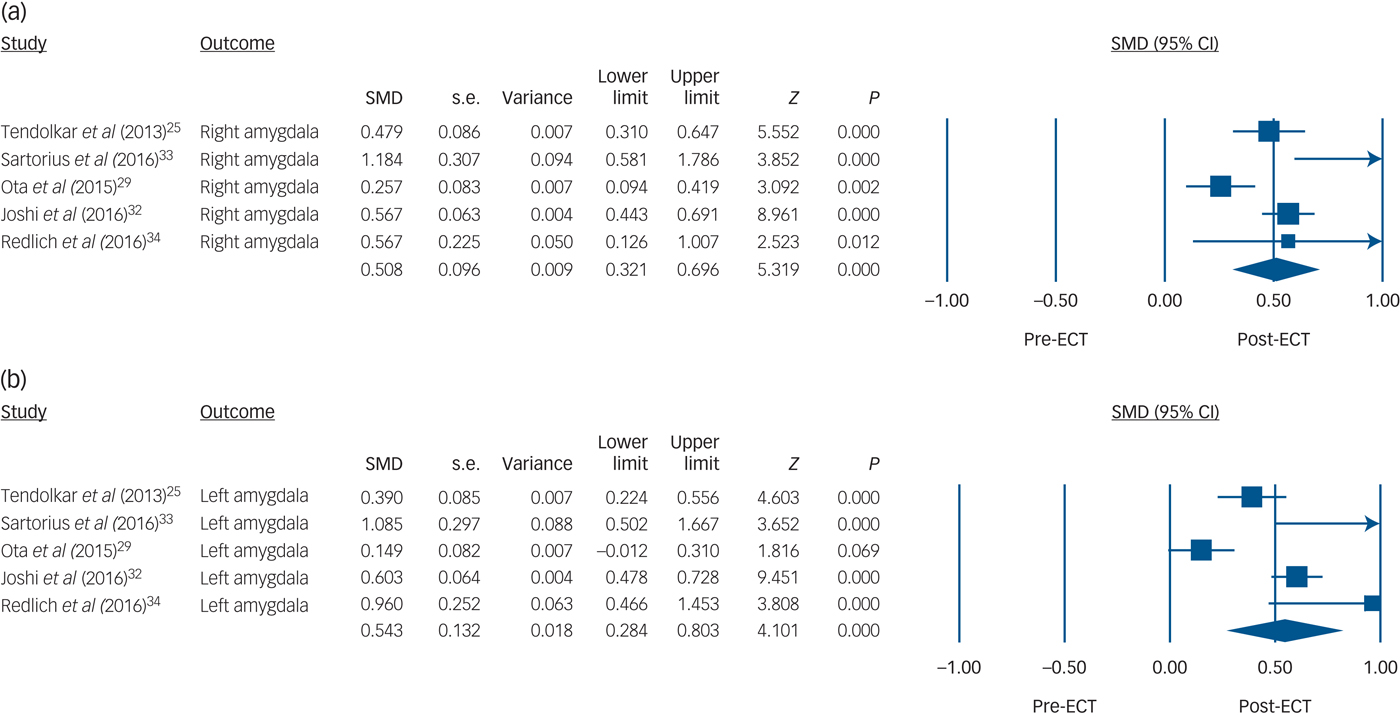
Fig. 2 Volume changes with electroconvulsive therapy (ECT) in (a) the right amygdala and (b) the left amygdala.
Subgroup analyses and meta-regression
A subgroup analysis for the left hippocampal volume comparing the unmedicated and medicated cohorts revealed that left hippocampal volume increased in both subgroups (medicated cohort: 6 studies, n = 149, 77%, SMD = 0.25, 95% CI 0.09 to 0.41, Z = 3.11; unmedicated cohort: 2 studies, n = 44, 23%, SMD = 0.58, 95% CI 0.33 to 0.83, Z = 4.62); however, the increase was more evident in the unmedicated group (P = 0.027) (online Fig. DS4). There was no significant difference in a subgroup analysis for the right hippocampus (P = 0.086). In the meta-regression analyses age was shown to have a negative correlation with left hippocampal volume increase after ECT (8 studies, n = 193, coefficient=−0.017, 95% CI −0.030 to −0.003, P = 0.01) (Fig. 3). Besides age, we also found a negative correlation between percentage of those who responded and percentage in remission with left hippocampal volume increase, although the number of studies for each meta-regression was small (7 studies, n = 178, coefficient = −0.013, 95% CI −0.025 to −0.0004, P = 0.04; 5 studies, n = 140, coefficient = −0.015, 95% CI −0.020 to −0.010, P < 0.01, respectively) (online Fig. DS5). Proportion of gender (P = 0.88), proportion of bipolar disorder (P = 0.72), proportion of RUL electrode placement (P = 0.50) and percentage reduction in depression severity score (P = 0.18) did not correlate with left hippocampal volume increase. In the meta-regression analyses of right hippocampal volume, age (P = 0.15), gender proportion (P = 0.42), proportion with bipolar disorder (P = 0.88), proportion of RUL electrode placement (P = 0.32), percentage reduction in depression severity score (P = 0.63), percentage responding (P = 0.62) and percentage in remission (P = 0.25) did not correlate.
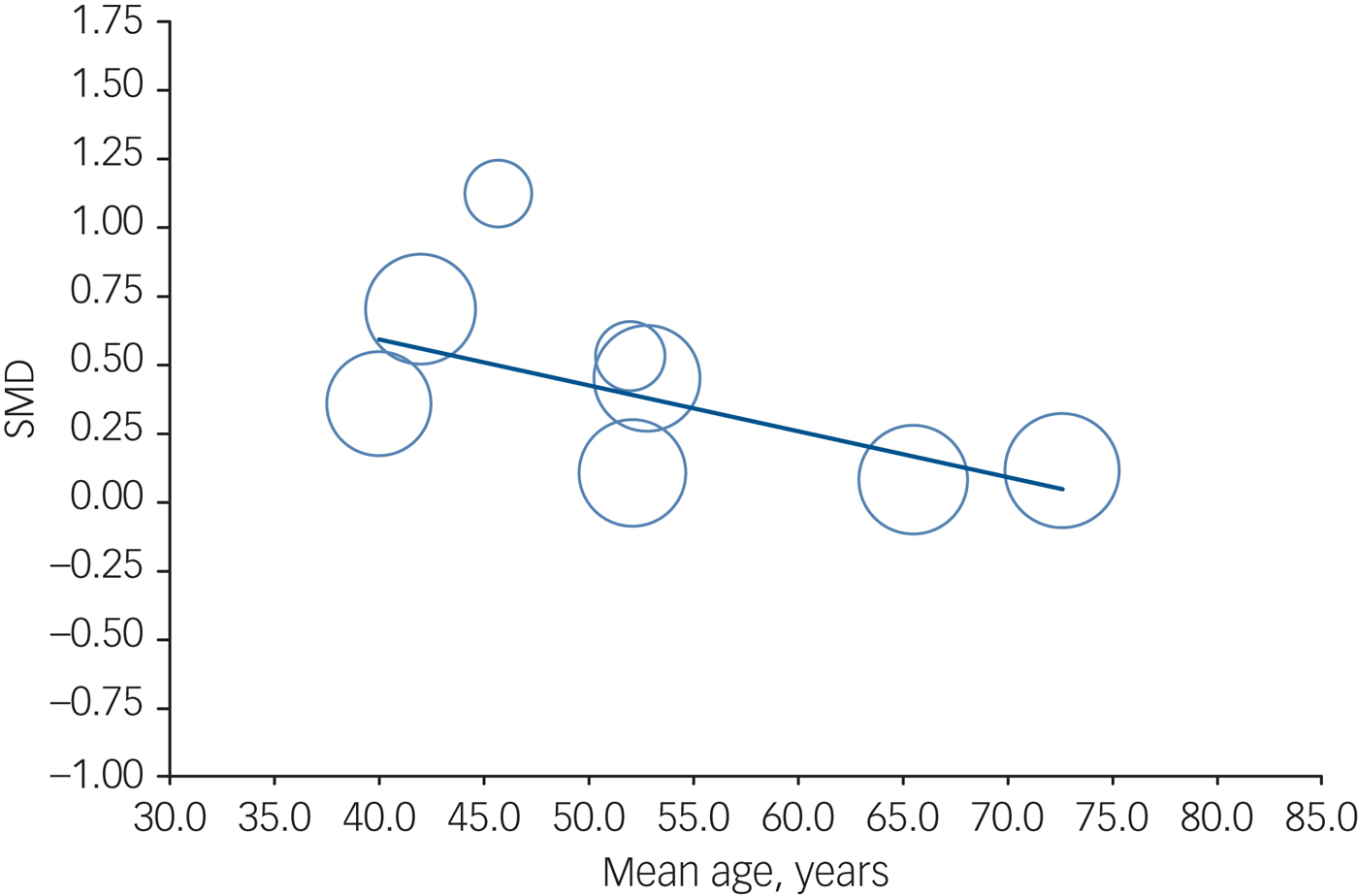
Fig. 3 Meta-regression result showing an association between age and volume changes with electroconvulsive therapy in the left hippocampus. Each study is represented as a circle, with larger circles symbolising greater sample sizes. The regression line is presented as a straight line. Note that studies including younger patients reported larger volume changes (P = 0.01).
Publication bias
The funnel plots to assess publication bias were asymmetrical (online Fig. DS6). Subsequently we used the trim and fill method to adjust for potential publication bias; imputing missing studies did not change the result.
Discussion
To the best of our knowledge this is the most up-to-date and largest meta-analysis investigating ECT-related brain structural changes. The main results of our study indicate that ECT is associated with increased bilateral hippocampal and amygdala volumes in depressed patients; volume increase in hippocampi was more evident in the younger cohort. Moreover, hippocampal volume changes were shown to be negatively associated with clinical improvement. Our meta-analysis incorporated the largest study published on this topic,Reference Bouckaert, Dols, Emsell, De Winter, Vansteelandt and Claes18 which had not been included in the earlier meta-analysis.Reference Wilkinson, Sanacora and Bloch17 Moreover, our meta-analysis further advanced the previous report by considering the amygdala in addition to the hippocampus, and analysed actual volume changes after obtaining data through contacting the authors. The question arises as to what underlying biological mechanisms account for hippocampal and amygdala volume changes with ECT. Although one might contend that volume changes with ECT are due to alterations in water content, there are several MRI and diffusion tensor imaging studies suggesting that oedema is unlikely to be the primary cause of the observed hippocampal volume changes with ECT.Reference Nordanskog, Dahlstrand, Larsson, Larsson, Knutsson and Johanson12, Reference Jorgensen, Magnusson, Hanson, Kirkegaard, Benveniste and Lee28, Reference Kunigiri, Jayakumar, Janakiramaiah and Gangadhar39, Reference Szabo, Hirsch, Krause, Ende, Henn and Sartorius40 On the other hand, reported volume changes from T 1-weighted imaging might be related to changes in blood flow because the T 1 relaxation times for arterial blood and grey matter are not clearly distinguishable.Reference Franklin, Wang, Shin, Jagannathan, Suh and Detre41 Neuroimaging measures themselves are difficult to relate to underlying biology, and animal studies at the cellular and molecular level can be more informative for explaining neuroimaging observations,Reference Zatorre, Fields and Johansen-Berg42 as discussed below.
Hippocampal volume
One possible explanation for hippocampal volume increase with ECT is neurogenesis in the hippocampus. According to previous preclinical studies, ECS increased the number of neurons in the rat dentate gyrus,Reference Madsen, Treschow, Bengzon, Bolwig, Lindvall and Tingstrom8, Reference Malberg, Eisch, Nestler and Duman9 and the increase was sustained for at least 3 months.Reference Madsen, Treschow, Bengzon, Bolwig, Lindvall and Tingstrom8 Not only was ECS-induced neurogenesis observed in the dentate gyrus of monkey hippocampus, but it was also more substantial than in rodents.Reference Perera, Coplan, Lisanby, Lipira, Arif and Carpio10 Interestingly, antidepressant medication also increased hippocampal neurons in both mice and non-human primates,Reference Santarelli, Saxe, Gross, Surget, Battaglia and Dulawa43, Reference Perera, Dwork, Keegan, Thirumangalakudi, Lipira and Joyce44 and selective disruption of neurogenesis in the dentate gyrus by irradiation blocked the behavioural effects of chronic administration of antidepressants.Reference Santarelli, Saxe, Gross, Surget, Battaglia and Dulawa43, Reference Perera, Dwork, Keegan, Thirumangalakudi, Lipira and Joyce44 Electroconvulsive shock is a more potent and faster stimulator of neurogenesis than antidepressants, and increases cell proliferation 2.5–4-fold compared with 1.5-fold by antidepressants;Reference Malberg, Eisch, Nestler and Duman9 furthermore, it can start neurogenic action within 3 days compared with 2–3 weeks with antidepressants.Reference Warner-Schmidt and Duman11 This evidence seems to be consistent with the clinical facts that ECT is a more potent and faster antidepressant treatment than medication. Hippocampal neurogenesis has also been observed in adult humans.Reference Eriksson, Perfilieva, Bjork Eriksson, Alborn, Nordborg and Peterson45, Reference Spalding, Bergmann, Alkass, Bernard, Salehpour and Huttner46 Hence, increased neurogenesis in the dentate gyrus might contribute to the hippocampal volume increases with ECT.
Along with neurogenesis, gliogenesis has been considered to be an important factor in the pathophysiology of depression and the effects of antidepressant treatment, including ECT. Electroconvulsive shock induces a proliferation of glial progenitor cells (NG2-positive cells),Reference Wennstrom, Hellsten, Ekdahl and Tingstrom47, Reference Wennstrom, Hellsten, Ekstrand, Lindgren and Tingstrom48 increases the number of glial cells,Reference Kaae, Chen, Wegener, Madsen and Nyengaard49 and changes the morphology and activation of glial cells.Reference Jansson, Wennstrom, Johanson and Tingstrom50 Since NG2-positive cells can have an important role in regulating synaptic plasticity and function,Reference Dou and Levine51 ECS may cause changes in synaptic structures and functions. In addition to elevating synaptic proteins,Reference Bolwig and Jorgen52 ECS also increases the total synapse number and volume of the dentate gyrus,Reference Chen, Madsen, Wegener and Nyengaard53 and has promoted the maturation of dendritic spines.Reference Zhao, Warner-Schmidt, Duman and Gage54 These changes seem to be important not only for volume changes in the hippocampus but also for the remodelling of the neural circuit that mediates the therapeutic effect of ECT. Additionally, angiogenesis can be an alternative explanation for volume increase: ECS increased endothelial cell numbers by up to 30% and vessel length by 16% in the molecular layer of the dentate gyrus.Reference Hellsten, West, Arvidsson, Ekstrand, Jansson and Wennstrom55 In vivo human positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies have also revealed increased perfusion and metabolism in the medial temporal lobe including the parahippocampal gyrus and hippocampus after ECT,Reference Takano, Motohashi, Uema, Ogawa, Ohnishi and Nishikawa56–Reference Reininghaus, Reininghaus, Ille, Fitz, Lassnig and Ebner59 which may reflect angiogenesis.
Amygdala volume
We found that ECT increased bilateral amygdala volume. Human post-mortem studies have revealed reduction in the density of total glia and oligodendrocytes in the amygdala in patients with major depressive disorder.Reference Bowley, Drevets, Ongur and Price60, Reference Hamidi, Drevets and Price61 According to an animal study, ECS increased the number of glial cells (oligodendrocytes, and microglia in non-activated state) in rat amygdala.Reference Wennström, Hellsten and Tingstrom62 Histological studies also showed a reduction in glial cells in patients with depression in the subgenual cingulate cortex,Reference Ongur, Drevets and Price63 an area in which two studies in our systematic review reported volume increases with ECT.Reference Dukart, Regen, Kherif, Colla, Bajbouj and Heuser26, Reference Ota, Noda, Sato, Okazaki, Ishikawa and Hattori29 Although speculative, based on this evidence ECT seems able to reverse pathological changes in glial cells in patients with depression, and this may account for volume increase in the amygdala with ECT.
Age and hippocampal volume changes
Our meta-regression analysis showed that ECT-induced hippocampal volume changes were age-dependent; the volume increase was more prominent in younger patients than in older ones, although this was observed only in the left hippocampus. This finding may be underpinned by findings from the following studies. Animal studies showed that old mice expressed markers for neither neural stem cells nor neuronal progenitors;Reference Rotheneichner, Lange, O'Sullivan, Marschallinger, Zaunmair and Geretsegger64 ECS increased the volume of dentate gyrus in younger mice but did not significantly increase its volume in 20-month-old mice.Reference Rotheneichner, Lange, O'Sullivan, Marschallinger, Zaunmair and Geretsegger64 In human studies, older adults showed low cell proliferation rate in dentate gyrus.Reference Czeh and Lucassen65 The reason why the age effect was only observed on the left side might be the sensitivity of left hemisphere to age-related volume changes.Reference Shan, Liu, Sahgal, Wang and Yue66
Medication and volume changes
Our subgroup analysis revealed that unmedicated patients had greater hippocampal volume increases than medicated patients. Previous preclinical,Reference Santarelli, Saxe, Gross, Surget, Battaglia and Dulawa43, Reference Perera, Dwork, Keegan, Thirumangalakudi, Lipira and Joyce44 post-mortem,Reference Boldrini, Santiago, Hen, Dwork, Rosoklija and Tamir67 and human cross-sectional and longitudinal studies,Reference Malykhin, Carter, Seres and Coupland68, Reference Frodl, Jager, Smajstrlova, Born, Bottlender and Palladino69 have consistently reported that antidepressant usage was associated with increased hippocampal volume. Additionally, a recent well-designed longitudinal study confirmed antidepressant-induced hippocampal volume increase.Reference Arnone, McKie, Elliott, Juhasz, Thomas and Downey70 In addition, aripiprazole and lithium, which are used for augmentation therapy in depressed patients, increase hippocampal volume.Reference Bodnar, Malla, Makowski, Chakravarty, Joober and Lepage71–Reference Hajek, Kopecek, Hoschl and Alda73 Hence, it is plausible that the medicated patients had a larger hippocampal volume than the unmedicated group at baseline (pre-ECT) so the changes of hippocampal volume with ECT were relatively small.
Electrode placement
Based on our meta-regression analysis, bilateral and unilateral stimulation equally increased hippocampal volume. One may speculate that generalised seizure, not electrical stimulation, might be a major contributor to volume changes with ECT. In contrast, studies using RUL placement and studies analysing whole-brain samples have consistently reported that volume increases were restricted to the right side.Reference Dukart, Regen, Kherif, Colla, Bajbouj and Heuser26, Reference Bouckaert, De Winter, Emsell, Dols, Rhebergen and Wampers30, Reference Sartorius, Demirakca, Bohringer, Clemm von Hohenberg, Aksay and Bumb33, Reference Redlich, Opel, Grotegerd, Dohm, Zaremba and Burger34 We did not include data from these whole-brain analyses because our meta-analysis focused only on hippocampus and amygdala volumes.
Clinical relevance of hippocampal changes
We found a negative correlation between age and the left hippocampal volume increase. Moreover, we found a negative correlation between the percentages of those responding and those in remission with left hippocampal volume increase. However, it seems that the age effect is stronger than that of the responder and remission groups, as the correlation coefficient was numerically the highest for age among these factors. Clinical studies have shown that elderly depressed patients respond to ECT better and faster than younger patients.Reference O'Connor, Knapp, Husain, Rummans, Petrides and Smith74–Reference Kellner, Husain, Knapp, McCall, Petrides and Rudorfer77 However, our results suggest that volume increase is smaller in older patient populations, who are more likely to have better response to ECT. The ECT-induced hippocampal volume changes were not associated with nor negatively correlated with clinical improvement. Therefore, the clinical relevance of hippocampal volume increase after ECT is still elusive. One possible explanation for the lack of association between hippocampal volume increase and clinical improvement could be that the brain's volume changes could be a byproduct of ECT treatment, and those not responding to ECT could show changes in hippocampal volume similar to those in the responder group.Reference Bouckaert, Dols, Emsell, De Winter, Vansteelandt and Claes18, Reference Nordanskog, Larsson, Larsson and Johanson24, Reference Tendolkar, van Beek, van Oostrom, Mulder, Janzing and Voshaar25, Reference Jorgensen, Magnusson, Hanson, Kirkegaard, Benveniste and Lee28 Another is that cognitive changes after ECT might be related to hippocampal volume changes. Unfortunately, we could not conduct a meta-regression to address the influence of cognition because only one study reported the relationship between hippocampal volume changes and changes in cognitive function.Reference Nordanskog, Larsson, Larsson and Johanson24 In order to better describe the moderators for brain volume increase, large studies including a wide range of patient ages and/or clinical improvements are needed. In addition, our results seem to be inconsistent with previous studies showing that hippocampal volume was associated with clinical response to antidepressant medications.Reference Phillips, Batten, Tremblay, Aldosary and Blier78, Reference Colle, Cury, Chupin, Deflesselle, Hardy and Nasser79 This inconsistency may lead to the notion that the hippocampal volume can be a biomarker for antidepressant medications but not for ECT. Future studies should investigate not only hippocampal volume changes but also other brain regional changes with ECT.
Limitations
Several limitations of this study should be noted. First, the number of studies in the meta-analysis was relatively small, and we excluded about half of the studies from systematic review owing to a lack of statistical values, which may have caused bias. Second, significant heterogeneity was found. However, the results of all studies were similar (i.e. volume increase) and the trim and fill method showed that the results did not change even if there were unpublished negative studies. In addition, there was variability across studies in how to conduct ECT (electrode placement), how to assess depression severity and how to assess volume (manual tracing and automated segmentation). These heterogeneities might limit our meta-regression analyses. A global research collaboration collecting data from each individual patient and conducting mega-analyses could overcome these limitations.Reference Oltedal, Bartsch, Sorhaug, Kessler, Abbott and Dols80
Further research
Electroconvulsive therapy increased hippocampal and amygdala volumes. Hippocampal volume changes were negatively associated with age and clinical improvement. However, the clinical relevance of hippocampal volume increase with ECT needs further investigation using larger studies.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2017.11









eLetters
No eLetters have been published for this article.