INTRODUCTION
Cannabis is the most widely used substance in the world (Johnston, O’Malley, Bachman, & Schulenberg, Reference Johnston, O’Malley, Bachman and Schulenberg2012; SAMHSA, 2011). Largely due to the primary psychoactive component, delta-9-tetrahydrocannabinoid (Δ-9-THC), cannabis generates psychotomimetic effects, cognitive dysfunction, psychomotor deficits, and increased risk for the development of schizophrenia, depression, and anxiety (Radhakrishnan, Wilkinson, & D’Souza, Reference Radhakrishnan, Wilkinson and D’Souza2014). Understanding the chronic effects of cannabis on cognition is necessary, especially as cannabis use is expected to increase (Carliner, Brown, Sarvet, & Hasin, Reference Carliner, Brown, Sarvet and Hasin2017; Hasin etal., Reference Hasin, Saha, Kerridge, Goldstein, Chou, Zhang and Smith2015), likely related to a decreasing perception of risk (Azofeifa, Reference Azofeifa, Mattson, Schauer, McAfee, Grant and Lyerla2016; Carliner etal., Reference Carliner, Brown, Sarvet and Hasin2017; Zehra etal., Reference Zehra, Burns, Liu, Manza, Wiers, Volkow and Wang2018) and liberalization of cannabis laws.
Both acute and chronic cannabis exposure have been associated with poorer neurocognitive performance in executive function, verbal learning and memory, attention, and psychomotor function, and these deficits have also been observed after periods of abstinence (Broyd, van Hell, Beale, Yuecel, & Solowij, Reference Broyd, van Hell, Beale, Yuecel and Solowij2016; Dahlgren, Sagar, Racine, Dreman, & Gruber, Reference Dahlgren, Sagar, Racine, Dreman and Gruber2016; Gruber & Sagar, Reference Gruber and Sagar2017; Hanson etal., Reference Hanson, Winward, Schweinsburg, Medina, Brown and Tapert2010; Messinis, Kyprianidou, Malefaki, & Papathanasopoulos, Reference Messinis, Kyprianidou, Malefaki and Papathanasopoulos2006; Ranganathan & D’Souza, Reference Ranganathan and D’Souza2006). Additionally, in a large prospective study, regular cannabis use (compared to a cannabis naïve baseline) was associated with declines in cognitive function, specifically in domains of intelligence, executive function, and processing speed (Meier etal., Reference Meier, Caspi, Ambler, Harrington, Houts, Keefe and Moffitt2012). It is important to acknowledge that there are mixed findings regarding the relationship between cognition and cannabis. For example, investigations using quasi-experimental twin-study designs have observed minimal relationships between cannabis and declines in cognition (Meier etal., Reference Meier, Caspi, Danese, Fisher, Houts, Arseneault and Moffitt2018; Ross etal., Reference Ross, Ellingson, Rhee, Hewitt, Corley, Lessem and Friedman2020). Also, a recent meta-analysis (Figueiredo, Tolomeo, Steele, & Baldacchino, Reference Figueiredo, Tolomeo, Steele and Baldacchino2020) suggested that cannabis use is associated with small effect sizes over a spectrum of cognitive measures, with the largest effect sizes for short-term (d = .48) and long-term (d = .43) memory deficits. A meta-analysis by Scott etal. (Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018) further indicated that the modest cognitive impairments shown by cannabis users are further diminished after abstinence longer than 72 h (Scott etal., Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018).
The mixed literature on the relationship between cognition and cannabis may in part be due to the failure to consider biological sex as a potential moderating variable. While historically the prevalence of cannabis use has been two to three times higher in males than females, in the 21st century, prevalence rates have been converging in the United States (Chapman etal., Reference Chapman, Slade, Swift, Keyes, Tonks and Teesson2017; Johnson etal., Reference Johnson, Fairman, Gilreath, Xuan, Rothman, Parnham and Furr-Holden2015; Nia, Mann, Kaur, & Ranganathan, Reference Nia, Mann, Kaur and Ranganathan2018). Clinical and preclinical data have observed differences in behavioral effects and development of tolerance to cannabis. These differences may be related to sex-dependent differences in cannabinoid metabolism and influence of sex-specific hormones and/or endocannabinoids (Fattore & Fratta, Reference Fattore and Fratta2010; Fratta & Fattore, Reference Fratta and Fattore2013; Ketcherside, Baine, & Filbey, Reference Ketcherside, Baine and Filbey2016; Nia etal., Reference Nia, Mann, Kaur and Ranganathan2018; Paola Castelli etal., Reference Paola Castelli, Fadda, Casu, Sabrina Spano, Casti, Fratta and Fattore2014; Prashad, Hammonds, Wiese, Milligan, & Filbey, Reference Prashad, Hammonds, Wiese, Milligan and Filbey2020). Further, few studies have systematically investigated sex differences as a moderator of the relationship between cannabis use and observed cognitive deficits. In acute challenge studies with the administration of cannabinoids, some studies failed to find an impact of biological sex on cognitive measures, such as impulsivity, selective and divided attention, cognitive flexibility, or time estimation (Anderson, Rizzo, Block, Pearlson, & O’Leary, Reference Anderson, Rizzo, Block, Pearlson and O’Leary2010; McDonald, Schleifer, Richards, & de Wit, Reference McDonald, Schleifer, Richards and de Wit2003). Others found that women appeared to be more sensitive to the acute effects of cannabinoids on psychomotor function (Roser etal., Reference Roser, Gallinat, Weinberg, Juckel, Gorynia and Stadelmann2009), while showing enhancement in spatial working memory (Makela etal., Reference Makela, Wakeley, Gijsman, Robson, Bhagwagar and Rogers2006).
Similarly, mixed findings have been observed regarding the relationship between sex, chronic cannabis use, and cognition. Chronic cannabis use has been associated with greater deficits in psychomotor and cognitive speed in males, compared to females, after one week of abstinence (Lisdahl & Price, Reference Lisdahl and Price2012). In contrast, greater visuospatial memory deficits were observed in females (Pope, Jacobs, Mialet, Yurgelun-Todd, & Gruber, Reference Pope, Jacobs, Mialet, Yurgelun-Todd and Gruber1997), which may be associated with age of initiation of cannabis use (Noorbakhsh, Afzali, Boers, & Conrod, Reference Noorbakhsh, Afzali, Boers and Conrod2020). Additionally, a series of studies by Crane and colleagues demonstrated that cannabis use was more strongly associated with deficits in episodic memory in females compared to males, although more cannabis use was correlated with poor decision-making in males, but not females (Crane, Schuster, & Gonzalez, Reference Crane, Schuster and Gonzalez2013; Crane, Schuster, Mermelstein, & Gonzalez, Reference Crane, Schuster, Mermelstein and Gonzalez2015). Crane etal. (Reference Crane, Schuster, Mermelstein and Gonzalez2015) also observed that in women, but not men, earlier onset of cannabis use was associated with greater memory deficits. Additionally, in women, earlier onset of cannabis use was associated with lower estimated intelligence, although this was observed at the trend (p = .10) level (Crane etal., Reference Crane, Schuster, Mermelstein and Gonzalez2015).
Neuroimaging investigations have also demonstrated sex differences on the neurobiological impact of regular cannabis use. For example, increased amygdala volumes and worse depression and anxiety have been observed in female users (McQueeny etal., Reference McQueeny, Padula, Price, Medina, Logan and Tapert2011). Also, group by biological sex interactions has been observed such that female cannabis users had larger prefrontal cortex volumes and males had smaller volumes compared to non-using peers (Medina etal., Reference Medina, McQueeny, Nagel, Hanson, Yang and Tapert2009). Electrophysiological investigations of early sensory processing revealed altered visual steady state evoked potentials in females, but not males, suggesting altered primary visual neural circuits in women with regular cannabis use (Skosnik, Krishnan, Vohs, & O’Donnell, Reference Skosnik, Krishnan, Vohs and O’Donnell2006). Finally, previous work from our laboratory using magnetic resonance spectroscopy suggested that glutamate levels may be dependent on recent cannabis use, and further that this relationship may be modulated by sex (Newman etal., Reference Newman, Cheng, Schnakenberg Martin, Dydak, Hetrick and O’Donnell2019).
While each of the studies investigating biological sex as a moderator of the relationship between cognition and cannabis use made unique scientific contributions, there remain gaps in the literature. For example, the literature would benefit from systematic comparison of current, regular cannabis users to non-using peers (a control group). While investigation after periods of abstinence is important, prolonged abstinence may represent a different cognitive state compared to regular, recent use, should any deficits recover with abstinence. The current study sought to address these limitations. This study investigated cognitive function in males and females with regular cannabis (CB) use compared to non-using healthy controls (HC). We hypothesized that i) the group with regular CB use would have decreased cognitive performance, compared to the HC group, especially in domains of memory, ii) cognitive performance would be moderated by sex, and iii) earlier age of onset of cannabis use and total lifetime exposure would be associated with worse cognitive performance.
METHODS
Subjects
Forty current cannabis users (CB; 22 female) and forty healthy controls (HC; 23 female) were recruited from the community with local advertisements and via word of mouth.
Subjects were determined to be free of any current Axis I disorder as per the Structured Clinical Interview for Diagnostic and Statistical Manual, 4th. Edition (DSM-IV)-TR (SCID), Research Version (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams2002), except cannabis abuse or dependence. Exclusion criteria for all subjects included lifetime dependence of any substance (excluding nicotine and cannabis). All participants were 18 years of age or older, and free of any neurological disorder, head injury with loss of consciousness greater than five minutes, learning disability, and family history of a first degree relative with psychosis. Those in the CB group had a current rate of cannabis use ≥ 1×/week for a minimum of the past 3 months. Subjects in the healthy control group were required to have no cannabis use in the past 3 months, no history of cannabis DSM-IV abuse or dependence, a negative urine screen, and lifetime total use of <16 exposures. All subjects also underwent urine toxicology to validate self-report regarding current CB use and verify abstinence from other illicit substances.
Procedures
Participants were recruited via local advertisements and word of mouth, to which they were instructed to contact the laboratory by phone for a brief phone screen to assess general eligibility. Potentially eligible subjects were invited into the laboratory for a full screening evaluation and study participation. All participants were asked to refrain from recent use of alcohol, cannabis, and other illicit substances from the evening prior to their scheduled test day (∼12 h before testing). After the informed consent process, subjects underwent a diagnostic clinical interview with the SCID, Research Version (First etal., Reference First, Spitzer, Gibbon and Williams2002). Subjects then completed a series of self-report measures assessing lifetime substance use. Eligible subjects were then administered tests of cognition. For details of cognitive assessments, see below. Total study participation took approximately 4–5 h. Subjects were compensated $12/hour for the initial interview, questionnaires, and cognitive testing.
Measures
Substance use
A Substance Use Questionnaire (SUQ), as used in previous studies by our laboratory (Fridberg, Vollmer, O’Donnell, & Skosnik, Reference Fridberg, Vollmer, O’Donnell and Skosnik2011; Skosnik etal., Reference Skosnik, D’souza, Steinmetz, Edwards, Vollmer, Hetrick and O’donnell2012), was used to collect information about many substances, including tobacco, caffeine, alcohol, cannabis, synthetic marijuana (i.e., spice, K2), sedatives/tranquilizers, ecstasy, speed, cocaine, opiates, hallucinogens, salvia divinorum, ketamine, phencyclidine, inhalants, and gamma-Hydroxybutyric acid. The SUQ also includes a fictitious substance, relevin, which if endorsed was used to exclude subjects for inaccurate over-reporting. None of the subjects in the study endorsed using this sham substance. The SUQ was used to determine the total lifetime exposures of cannabis use (number of occasions in which an individual used cannabis), age of cannabis initiation, number of occasions of uses in the past month (recent use), days since last use of cannabis, if subjects ever used wax, and if they used wax, the number of occasions in which wax was used over the past 6 months. Additionally, subjects were asked to report their last use all substances, including cannabis, alcohol, tobacco, and caffeine.
Cognition
Intellectual function was assessed using the full-scale intelligence quotient (IQ) derived from the Vocabulary and Matrix Reasoning subtests from the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, Reference Wechsler1987). Psychomotor performance was measured with the total score from the Digit Symbol Test from the Wechsler Adult Intelligence Scale III (WAIS III) (Tulsky, Ivnik, Price, & Wilkins, Reference Tulsky, Ivnik, Price and Wilkins2003). Verbal working memory was assessed with the Digit Span from the Wechsler Adult Intelligence Scale III total score measured by the correct number of trials summed between the forward and backward blocks. The Hopkins Verbal Learning Task – Revised (HVLT-R) (Benedict, Schretlen, Groninger, & Brandt, Reference Benedict, Schretlen, Groninger and Brandt1998) assessed verbal learning. In the HVLT-R, subjects were read a list of 12 words and then asked to repeat the word list back over a series of 3 trials. The sum of the three trials represented the total immediate recall score. After a 20-minute delay, subjects were asked to recall as many words as they could remember from the list, which was the delayed recall total score. A forced choice selection list was then read to subjects with the instructions to indicate words that were or were not on the list. The total correct number of hits was the cued recognition recall score. The retention percentage t-score was then determined using the scoring appendix, based on the ratio of words retained between the higher score on trials 2 or 3 and the total delayed recall. Finally, a recognition discrimination index t-score was also determined using the scoring appendix, based on the ratio of the number of true positives and false positives identified during cued recognition recall.
Statistical Analysis
All statistical analyses were performed with SPSS statistical software from IBM (CORP, Reference Corp2020). The Student’s t-test was used to assess for differences in age, BMI, and education. A chi-square test was used to assess for differences in biological sex, ethnicity, and race between cannabis users and controls (Table 1) and between male and female cannabis users (Table 2).
Table 1. Demographic information

SD = standard deviation.
* Race breakdown: Caucasian: Black/African American: Asian: American Indian/Alaska Native: Native Hawaiian or Other Pacific Islander: More than one race: Unknown; H = Hispanic; NH = not Hispanic; UK = unknown.
Table 2. Demographic features: male and female cannabis use groups
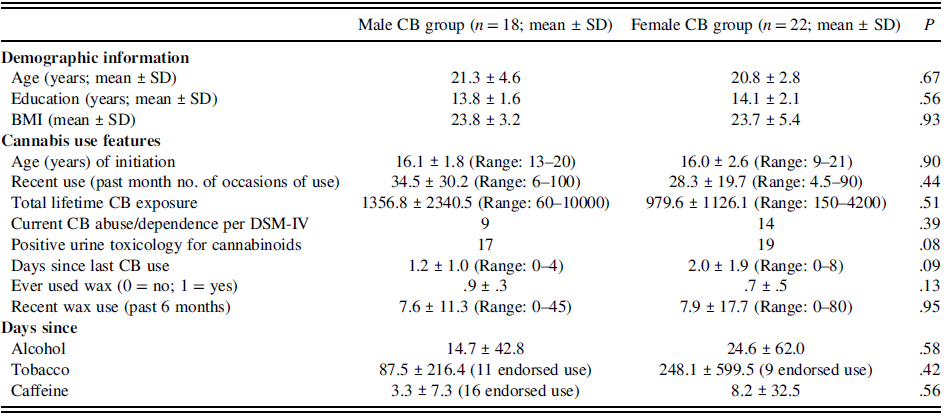
CB = cannabis; SD = standard deviation.
* p < .05.
Two-way analysis of covariance (ANCOVA) was used to assess group differences and interactions between biological sex and cannabis use. Education was used a covariate across analyses. In the presence of significant interaction effects between sex and group, the education-adjusted scores from the ANCOVA were submitted to post-hoc testing with Sidak adjustments to correct for multiple comparisons. Effect sizes of post-hoc group comparisons were measured with Cohen’s d. A Cohen’s d ≤ .2 was considered small, ≤.4 medium, and ≤.8 large (Cohen, Reference Cohen1988). To limit the number of tests performed, we also conducted one MANCOVA (controlling for education) with the five HVLT outcomes and findings remained consistent with the results presented below. Finally, for cognitive domains in which a significant main or interaction effect was observed on a cognitive measure, exploratory partial correlations controlling for education were used to assess associations between cognitive measures and features of cannabis use, including total lifetime exposure, age of initiation, time since last use, recent (past month) use, lifetime wax (high-concentration THC) exposure, and wax use over the past 6 months.
RESULTS
Subjects
As per independent samples t-tests and chi-square testing, groups did not differ in age (CB = 21.1 ± 3.7 years; HC = 22.7 ± 4.4 years), sex (CB = 18 males: 22 females; HC = 17 males: 23 females), race, or BMI (Table 1). Groups did significantly differ in years of completed education (p = .002). CB and HC groups did not differ in time since last use of alcohol, tobacco, or caffeine (p > .05). CB and HC groups did not differ in number of cigarettes per day (HC = .001 ± .01; CB = .74 ± 2.66). Although groups differed in number of alcoholic drinks over the past week (HC = 2.34 ± 3.78; CB = 5.81 ± 7.65; F(72) = 3.9, p = .02), groups did not differ on the Short-Michigan Alcohol Screening Test (SMAST), a measure of problematic drinking (p > .05). Controlling for number of drinks in the last week, in addition to education, did not change presented study findings which only controlled for education. More than half of the subjects (23/40) in the CB group met criteria for a DSM-IV lifetime diagnosis of CB abuse or dependence. Data for two participants in the CB group were insufficient to make a definitive diagnostic determination regarding CB abuse or dependence. Male and female CB use groups did not differ in their features of CB use (Table 2). Thirty-six subjects in the CB group had a positive urine toxicology for cannabinoids and zero in the HC group. Notably, groups did not differ in number of cigarettes per day, drinks over the past week, or in problematic drinking based on the SMAST (p > .05).
Significant differences were observed between the cannabis and control groups by sex on measures of intellectual function, psychomotor function, and verbal learning and memory (Table 3; Figures 1 and 2).
Table 3. Cognitive measures by group

HC = healthy control group; CB = cannabis use group.
Note: p = significance value from post-hoc pairwise comparisons in cases with significant interactions between group and biological sex; p = ns is indicative of domains in which post-hoc testing was not completed due to non-significant group × biological sex interactions; ![]() dark gray shading indicates when the cannabis group performed below the same-sex control group;
dark gray shading indicates when the cannabis group performed below the same-sex control group; ![]() the light gray shading indicates when the cannabis group performed better than the same-sex control group. All scores are the total/raw scores except for the WASI IQ and where indicated as a T-Score (i.e., retention percent).
the light gray shading indicates when the cannabis group performed better than the same-sex control group. All scores are the total/raw scores except for the WASI IQ and where indicated as a T-Score (i.e., retention percent).
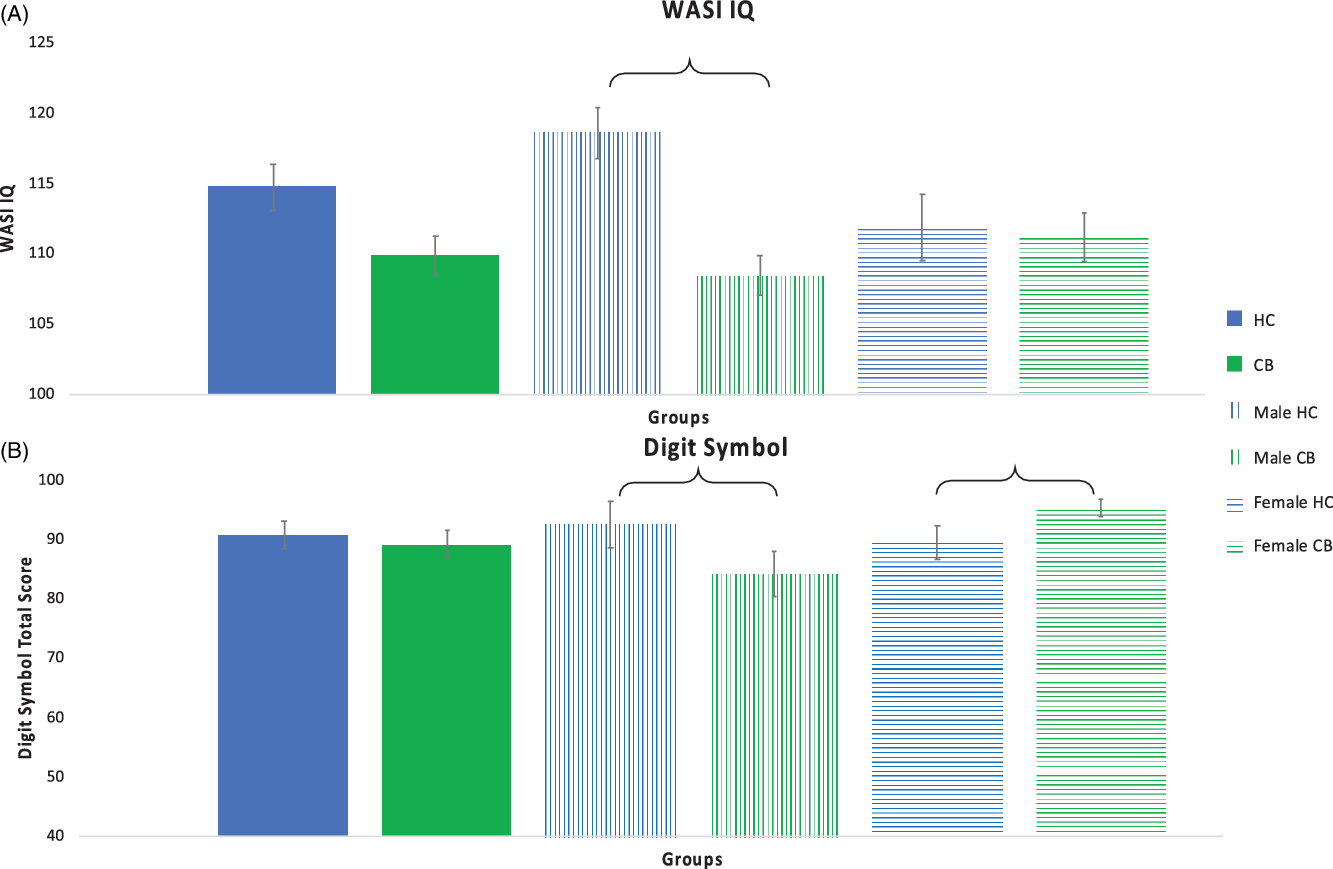
Fig. 1. Intellectual and Psychomotor Function. Intellectual function as measured with the WASI IQ (Panel A) and psychomotor function as measured with the Digit Symbol (Panel B). Significant differences between groups are indicated with a bracket. Error bars shown with standard error.
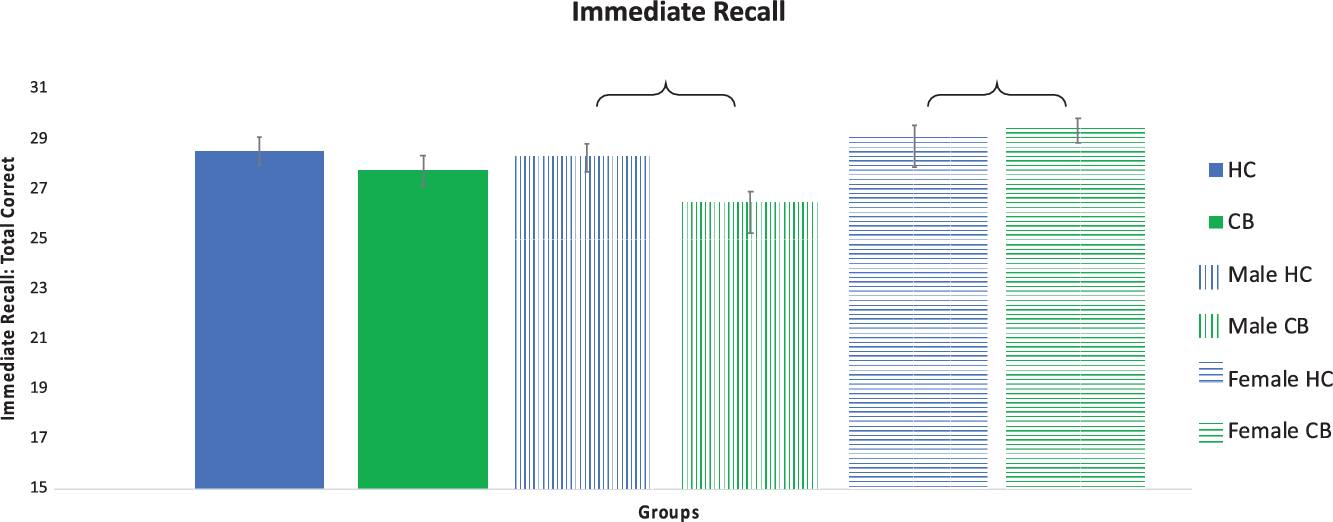
Fig. 2. Verbal Learning and Memory. Verbal learning and memory as measured with the Hopkins Verbal Learning Test (HVLT) total immediate recall. Significant differences between groups indicated with a bracket. Error bars shown with standard error.
WASI IQ: There was trend for a main effect of group on WASI IQ (F(1, 75) = 3.642, p = .060) but not for biological sex (F(1,75) = 1.078, p = .302). There was a significant interaction between group and biological sex (F(1,75) = 5.303, p = .024), such that the male CB group performed worse than male HCs. Post-hoc testing revealed that the male CB group was observed to have significantly lower scores compared to the male HC group (p < .001). There was no statistically significant difference between the two female groups (p = .797). Measures of effect sizes between the HC and CB groups, male groups, and female groups were Cohen’s d = .50, 1.18, and .069, respectively.
Digit Symbol: Main effects for group (Cohen’s d = .11) and sex were not significant (p > .05). There was a significant interaction between group and biological sex (F(1,75) = 5.204, p = .009), such that the male CB group performed worse than male HCs and the female HCs performed worse than the female CB group. Post-hoc testing revealed that the male CB group had significantly lower scores compared to the male HC group (p < .001; Cohen’s d = .68). Additionally, the female CB group was observed to have significantly higher scores compared to the female HC group (p < .001; Cohen’s d = .50).
Digit Span: Main effect of group was not significant (p > .05). There was a significant main effect of biological sex (F(1,75) = 4.766, p = .032), indicating that females had higher scores than males. The interaction between group and sex was not significant (p > .05). Measures of effect sizes between the HC and CB groups, male groups, and female groups were Cohen’s d = .14, .50, and .08, respectively.
HVLT: Total Recall: immediate and delayed: There were no main effects of group on total immediate recall (HC vs CB Cohen’s d = .21) or total delayed free recall (HC vs CB Cohen’s d = .41). There was a main effect of biological sex for total immediate recall (F(1,75) = 5.941, p = .017), but not for total delayed free recall (F(1,75) = 2.255, p = .137). A significant interaction between group and biological sex was observed for total immediate recall (F(1,75) = 3.955, p = .050) and total delayed free recall (F(1,75) = 7.006, p = .010). Post-hoc testing for total immediate recall demonstrated that the male CB group recalled significantly fewer words than the male HC group (p < .001; Cohen’s d = .77), whereas female CB users performed better than female controls (p = .02; Cohen’s d = .17). Post-hoc testing for delayed free recall demonstrated that the male CB group recalled significantly fewer correct responses compared to the male HC group (p < .001; Cohen’s d = 1.03), while the two female groups did not differ (p > .05; Cohen’s d = .08).
HVLT: Cued Recognition Recall: “hits” forced choice: The main effect of group was not significant (HC vs CB Cohen’s d = .07). There was a main effect of biological sex (F(1,75) = 5.839, p = .018), indicating that females had greater cued recognition recall than males. The interaction between group and biological sex was also not significant (p > .05). Measures of effect sizes between male groups and female groups were Cohen’s d = .27 and .27, respectively.
HVLT: Retention % [(delayed recall (trial 4)/higher score of trials 2 or 3) × 100] T-Score: Main effects of group (HC vs CB Cohen’s d = .32) and biological sex were not significant (p > .05). A significant interaction between group and biological sex (F(1,75) = 4.523, p = .037) was observed. Post-hoc testing revealed that the male CB group showed lower retention compared to the male HC group (p < .001; Cohen’s d = .79), while the two female groups did not differ (p > .05; Cohen’s d = .11).
HVLT: Recognition Discrimination Index (Total no of true positives-total no of false-positives): The main effect of group was not significant (HC vs CB Cohen’s d = .24). A significant main effect was observed for biological sex (F(1,75) = 9.854, p = .002). The interaction between group and sex was not statistically significant (p > .05). Measures of effect sizes between the male groups and female groups were Cohen’s d = .50, and .11, respectively. Notably, groups were not significantly different in their recognition discrimination index (RD) scores (p > .05). Only one participant (CB group) had an RD = 7, two (one in each of the CB and HC groups) had a score of 9, while all others were 10+. Thus, these RD scores were considered as indication of credible responding by study participants (Abeare etal., Reference Abeare, Hurtubise, Cutler, Sirianni, Brantuo, Makhzoum and Erdodi2020; Bailey, Soble, Bain, & Fullen, Reference Bailey, Soble, Bain and Fullen2018; Sawyer, Testa, & Dux, Reference Sawyer, Testa and Dux2017).
Relationship between observed cognitive performance and features of cannabis use: For all cannabis use group participants (male CB + female CB), the only observed significant correlation was a negative association between digit symbol and total lifetime cannabis exposure (r = −.443, p = .007; Table 4), suggesting that increased lifetime cannabis use is associated with worse psychomotor function and processing speed.
Table 4. Correlations between cognitive measures with observed group differences and features of cannabis use: combined cannabis groups

WASI IQ = measure of intellectual function; HVLT = Hopkins Verbal Learning Test.
Note: Bold indicates significant p value (p < .05).
DISCUSSION
As hypothesized, cognitive performance was moderated by sex. Males, but not females, who were using cannabis at the time of study participation had worse performance on measures of intelligence, psychomotor speed, and immediate and delayed verbal recall, compared to same-sex non-using peers. Additionally, the female cannabis group performed significantly better on measures of psychomotor function and immediate verbal recall, compared to their female non-using peers. Among the observed cognitive deficits, only psychomotor function (digit symbol total score) was associated with a feature of cannabis use, i.e., total lifetime cannabis use. Significantly, the study failed to detect a main effect of cannabis use on all measures of cognition studied and thus highlighted the necessity of considering biological sex as a possible moderator of the impact of cannabis on the brain. This conclusion is further bolstered by sex differences that have been observed in neuropsychological performance more broadly (Asperholm, Högman, Rafi, & Herlitz, Reference Asperholm, Högman, Rafi and Herlitz2019). Notably, while differences on measures of cognition were observed, the degree of impairment did not reach a clinically impaired range, such as the level of impairment that is observed in major neurological disorders (i.e., stroke, dementia).
Causality cannot be inferred due to the cross-sectional nature of the study. Therefore, there are several possible explanations for the study findings. First, individual differences in cognitive performance may increase the likelihood of the initiation of cannabis use. This interpretation is supported in that the features of cannabis use, such as age of cannabis initiation and time since last use, were limited in their association with observed decreases in cognitive performance.
A second explanation is that cannabis use causes decreased cognitive performance in males and that women are less sensitive to the cognitive effects of cannabis. For example, while the female groups failed to differ on intellectual function, the male cannabis group was observed to have a 10-point deficit compared to the male healthy control group. These findings align with work by Lisdahl and Prince (2012), who observed neurocognitive deficits more pronounced in males, compared to females, after a week of abstinence from cannabis (Lisdahl & Price, Reference Lisdahl and Price2012). Also, findings align with longitudinal investigations by Meier etal. in which decreases in full-scale IQ were observed in cannabis users, both before and after controlling for years of education (Meier etal., Reference Meier, Caspi, Ambler, Harrington, Houts, Keefe and Moffitt2012). Findings also converge with those from experimental designs, collapsing across sex, involving the acute administration of cannabinoids in which acute THC administration caused significantly decreased immediate verbal recall (D’Souza etal., Reference D’Souza, Perry, MacDougall, Ammerman, Cooper, Wu and Krystal2004).
THC is generally lipophilic and stored in fat cells (Huestis, Reference Huestis2007; Kreuz & Axelrod, Reference Kreuz and Axelrod1973). Since females generally have greater body fat compared to men (Votruba & Jensen, Reference Votruba and Jensen2007), more cannabis may be retained by fat cells of the body instead of penetrating the brain, as suggested previously by Davis and Fattore (Davis & Fattore, Reference Davis, Fattore, Campolongo and Fattore2015). Additionally, it is possible that metabolism of cannabis metabolites may differ in males and females such that males are more susceptible to some of the effects of cannabis (Fattore & Fratta, Reference Fattore and Fratta2010). Supporting this view is the finding that the male and female cannabis groups did not differ on features of cannabis use, such as time since last use, use over the past month or time since initiation. Further, no significant differences were observed between the female cannabis and female healthy control groups on measures of intelligence, delayed recall, retention, and discrimination recognition. Notably, compared to non-using peers, the female cannabis use group had significantly better performance on measures of psychomotor speed and immediate verbal recall with medium effect sizes. It is important to acknowledge that stage of menstrual cycle was not assessed in this study. Therefore, it is possible that differences in menstrual cycle influences cognitive performance as has been observed in non-cannabis using females (Souza, Ramos, Hara, Stumpf, & Rocha, Reference Souza, Ramos, Hara, Stumpf and Rocha2012). Additionally, cannabis appears to alter the regulation of the hypothalamic-pituitary-ovarian axis which could impact the endocannabinoid system and cognitive performance (Brents, Reference Brents2016; Gorzalka & Dang, Reference Gorzalka and Dang2012). Future research is needed to clarify the relationship between menstrual cycle, cognition, and cannabis use.
There were two unexpected findings. First, a main effect of group was not observed on any of the included cognitive measures. It is possible that collapsing across biological sex causes a washout of any potential deficits, as evident by the group by biological sex interaction. This, along with the underrepresentation of female participants in research, may also be responsible for the inconsistent observations in the literature regarding the impact of regular cannabis use on cognition.
A second unexpected finding was that the female cannabis group performed significantly better on measures of psychomotor function and immediate verbal recall (total immediate recall) compared to their female non-using peers. In a previous study, which included a subset of samples in this analysis, females were observed to have greater levels of total N-acetylaspartate (NAA), a marker of functioning neurons, although the interaction between cannabis use and sex did not reach significance (Newman etal., Reference Newman, Cheng, Schnakenberg Martin, Dydak, Hetrick and O’Donnell2019). Additionally, while a different domain of working memory, previous work by Makela and colleagues (2006) demonstrated similarly increased performance on spatial working memory in women, but not men, with the acute administration of THC (Makela etal., Reference Makela, Wakeley, Gijsman, Robson, Bhagwagar and Rogers2006). Also, in both females and males, Crane etal. observed earlier age of cannabis onset was associated with better decision-making (Crane etal., Reference Crane, Schuster, Mermelstein and Gonzalez2015).
There are several important limitations to acknowledge about this study. Given the cross-sectional nature of the study, a causal link between the observed cognitive deficits and cannabis use could not be determined. Future research would benefit from longitudinal and twin-study designs with larger sample sizes. This study also did not evaluate the influence of psychiatric comorbidity or subthreshold symptoms for mental health disorders. Therefore, findings may not generalize to samples with older long-term users or users with comorbid and/or subthreshold psychiatric disorders. Population samples indicate very high lifetime rates of psychiatric comorbidity, approaching 90%, in persons with cannabis dependence (Agosti, Nunes, & Levin, Reference Agosti, Nunes and Levin2002), suggesting the necessity for future research to investigate the relationship between psychiatric comorbidity and cognition. This study also evaluated past week alcohol use and the average number of cigarettes consumed per day at the time of study participation. It would be important for future research to explore longer periods of time prior to study participation in regard to the influence of cigarette and alcohol use. This study attempted to evaluate the extent study participants were exposed to high THC content material through the inclusion of “wax”. Future research would be enhanced by the identification of biomarkers sensitive to remote use, and types of cannabis used by participants. Future research would also benefit from using updated measures, such as the DSM-5. This study also had a high heterogeneity of frequency of cannabis use in both of the cannabis use groups. It is possible that frequent cannabis use is associated with blunted neurocognitive effects compared to infrequent users (D’Souza etal., Reference D’Souza, Ranganathan, Braley, Gueorguieva, Zimolo, Cooper and Krystal2008). Thus, future studies should more systematically investigate the interaction between biological sex and disordered versus infrequent use. Finally, this study was powered to detect only moderate to large effect sizes, and therefore it is imperative that future research build on these findings with larger sample sizes.
Despite these limitations, the study findings suggest that there are sex-dependent cognitive impairments associated with cannabis use. Specifically, worse cognitive performance was most robustly observed in the male cannabis use group on measures of intellectual function, psychomotor speed, and verbal learning and memory. Further, in light of the increasing rates of cannabis use and changes in public policy, these findings highlight the important complexities in the relationship between cannabis and cognition.
ACKNOWLEDGEMENTS
The authors would like to thank the laboratory coordinators Karen Gomez Lorite, Leah Moravec, and Eli Calkins of the Clinical and Cognitive Neuroscience Center for their assistance with study recruitment, assessment, and data management.
FINANCIAL SUPPORT
This work is based upon work supported by the National Institute of Drug Abuse (NIDA; 5R21DA035493, BFO and SDN; R01DA048012 to WPH), the National Institute of Mental Health (NIMH; 2R01MH074983, WPH), a National Science Foundation (NSF) Graduate Research Fellowship (Grant #: 1342962, AMSM) as well as a NIDA T32 Predoctoral Fellowship (T32DA024628, AMSM; PI: Rebec). This research is also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Department of Veterans Affairs New England Mental Illness Research, Education, and Clinical Center (MIRECC), and VA Connecticut Healthcare System. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of NIDA, NIMH, NSF or the Department of Veterans Affairs.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
ETHICAL STANDARDS
After detailed explanation of research procedures, subjects were asked to provide verbal and written informed consent. Study procedures were approved by the Indiana University Institutional Review Board for protection of human subjects and according to guidelines of the Declaration of Helsinki.








