The metabolic syndrome (MetS) is considered a multiplex risk factor for atherosclerotic CVD and type 2 diabetes(Reference Grundy1). Major drivers of MetS are insulin resistance, atherogenic dyslipidaemia, prothrombotic state, elevated glucose, elevated blood pressure (BP), pro-inflammatory state and excess energy intake and concomitant obesity(Reference Grundy2). Mounting evidence suggests that lifestyle interventions (e.g. intermittent fasting and energetic restriction(Reference Sundfør and Svendsen3) or a weight reducing diet(Reference Harvie, Wright and Pegington4)) and lifestyle modifications (e.g. physical exercise(Reference Dalle Grave, Calugi and Centis5)) can reverse metabolic risk factors.
Ramadan is the ninth month of the Islamic lunar calendar during which healthy adult Muslims refrain from consuming food and drink from dawn to sunset. During Ramadan, the majority of Muslims throughout the world have two main meals, one immediately after sunset (suhoor) and the other just before dawn (iftar). During the night hours from sunset to dawn, people are allowed to eat and drink freely but may not consume any food or drink after dawn(Reference Sakr6). Ramadan diurnal intermittent fasting (RDIF) represents a unique fasting pattern that involves consistent diurnal abstinence from food and drink, including water, for a fasting period of 12–18 h (depending on the season) over 29–30 d. In the last two decades, several published systematic reviews and meta-analyses(Reference Sadeghirad, Motaghipisheh and Kolahdooz7–Reference Ziaee, Razaei and Ahmadinejad13) and original research studies have investigated the impact of RDIF on various health outcomes, including risk factors for the MetS, such as body weight, body fat, lipid profile and inflammatory and oxidative stress states. The ultimate complications of the MetS, such as CVD, have also been well-investigated. However, no published works have discussed or systematically analysed the MetS components as a cluster of factors involved in the etiopathogenesis of the syndrome.
This systematic review and meta-analysis aimed to systematically summarise and analyse available scientific evidence and clarify the results of published studies regarding the impact of RDIF on the MetS components among non-diabetic, non-athletic, healthy people aged 15 years and older, who observed Ramadan fasting. The MetS components investigated in this review were elevated waist circumference (WC), elevated TAG, reduced HDL-cholesterol, elevated fasting glucose (FG) and elevated BP(14).
The results of this analysis will expand knowledge regarding the metabolic impacts of RDIF and help to contextualise existing knowledge by examining all similar studies. The analysis of all available valid evidence pertaining to the effect of RDIF on metabolic outcomes will provide the best estimates of effect(Reference Freemantle and Geddes15). This analysis also aimed to clarify the variability between different observational and clinical studies on this topic(16). In addition, subgroup analyses for specific MetS components were performed to explore differences in findings among countries. The findings of the present review will help to determine the generalisability of the results of identified studies, direct future researchers towards knowledge gaps that need further examination using different research models (e.g. experimental interventional trials and animal models) and inform further subgroup analyses (as appropriate).
Materials and methods
This meta-analysis used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses as a guideline for reporting the findings(Reference Stroup, Berlin and Morton17).
Database searches
Two authors (A. A. O. and M. E. F.) conducted an electronic search on the CINAHL, Cochrane, EBSCOhost, Google Scholar, ProQuest Medical, PubMed/MEDLINE, ScienceDirect, Scopus and Web of Science databases for relevant studies published from 1950 to March 2019. The search strategy included the keywords: ‘Ramadan fasting’ OR ‘Ramadan diurnal fasting’ OR ‘Ramadan intermittent fasting’ OR ‘Ramadan model of intermittent fasting’ OR ‘Ramadan fast’ OR ‘intermittent prolonged fasting during Ramadan’ AND ‘metabolic syndrome’ OR ‘cardiometabolic risk factors’ OR ‘body composition’ OR ‘anthropometrics’ OR ‘waist circumference’ OR ‘fasting glucose’ OR ‘lipid profile’ OR ‘blood lipids’ OR ‘TAG’ OR ‘HDL’ OR ‘blood pressure.’ Reference lists of identified studies were searched to find additional articles and reviews to ensure that all relevant publications were included in this review.
Inclusion criteria
We included observational and interventional clinical studies that examined the impact of RDIF on the MetS components. The principal criteria for study inclusion and outcomes were the MetS components as reported in the International Diabetes Federation Consensus Worldwide Definition of the Metabolic Syndrome(14). Specific inclusion criteria for study selection were: (1) publication date between 1950 and March 2019; (2) original research articles published in the English language; (3) studies that reported numerical values (e.g., arithmetic mean with/without standard deviation (sd)) for at least one MetS component (WC, FG, TAG, HDL and systolic BP (SBP)); (4) studies that assessed the impact of RDIF on healthy people as the target population in prospective observational studies or on healthy controls in case–control, semi-experimental and experimental/interventional studies. We focused on studies that examined the effect of RDIF on the MetS components; therefore, we included studies that examined these components at least twice: pre-fasting as the baseline (e.g. few days or 1–2 weeks before Ramadan month or the first few days of Ramadan month), and post-fasting (at least 2 weeks into Ramadan month or after completion of Ramadan month). It should be noted that Islamic laws pertaining to fasting specify that premenopausal women are exempt from fasting during menstruation days; therefore, these women are not expected to complete fasting for the whole month of Ramadan. A similar exemption applies to elderly people who may find it hard to complete the whole Ramadan month and may miss some fasting days.
In all of the included studies, fasting glucose/lipid parameters were obtained from venous blood sample collected after 8–12 h of overnight fast, for the assay of all standard biochemical parameters included in glucoses/lipids profile. For the purpose of data analyses, all parameters were unified to mmol/l rounded to two decimal places.
Exclusion criteria
Identified articles were assessed against specific exclusion criteria to eliminate potential methodological and quality issues: (1) studies that exclusively involved fasting children and adolescents <18 years of age; (2) studies that included patients with different diseases or conditions (including diabetes) who observed RDIF; (3) studies on the impact of RDIF on Muslim athletes that observed Ramadan fasting; (4) studies with no available full-text, even after contacting the authors; (5) studies that expressed changes in the MetS components using bar graphs and curves without reporting exact numerical values; (6) studies involving pregnant or lactating women who observed Ramadan fasting; (7) studies that reported post-Ramadan measurement after one or more months, as evidence suggests RDIF-induced biochemical variables disappear/return to pre-fasting levels after 1 month of Ramadan month cessation(Reference Faris, Kacimi and Al-Kurd18–Reference Meo and Hassan20); (8) case reports, abstracts, review articles, editorials and non-English-language articles and (9) unpublished, non-peer-reviewed data. Articles that met any of these criteria were excluded from the present analysis (Fig. 1).
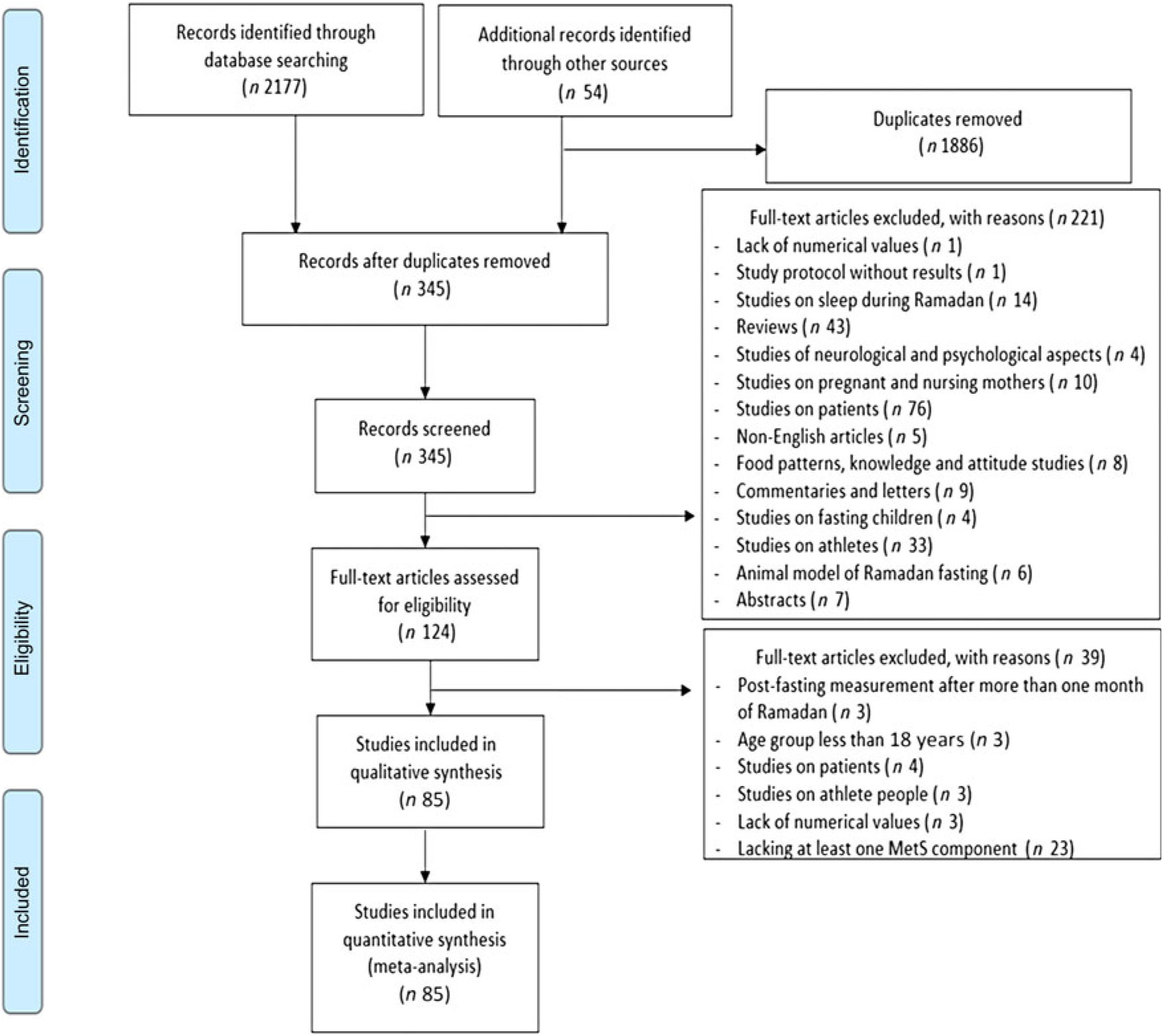
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart for the selection of publications included in the systematic review and meta-analysis. MetS, metabolic syndrome.
Main outcomes and measures
The principal outcome of this review was to report the effect of RDIF on effect size changes in the MetS components (WC, FG, TAG, HDL and SBP). SBP was chosen as it is a major component of BP. Two authors (A. A. O. and M. E. F.) independently screened the titles and abstracts of identified studies to assess the studies for eligibility. The first step of screening was examining all titles and abstracts to exclude irrelevant publications. Two authors (M. E. F. and J. A.) performed this initial screening, which was validated by another author (A. A. O). Any conflicts in opinions regarding study eligibility were resolved through dialogue with a fourth author (H. A. J.) to reach consensus. To standardise data extraction, the review team systematically collected and coded data for study characteristics (e.g. title, year, country, sample size and participants’ characteristics such as age, sex or proportion of males) and the main findings for the MetS components before and after RDIF.
Estimating fasting time length
Ramadan month as presented in the lunar calendar was matched with the Gregorian calendar using a time and date website (https://www.timeanddate.com/holidays/us/ramadan-begins). The daily length of fasting during Ramadan month was calculated using the sunrise and sunset times reported for that month for the city/country of each included study (https://www.timeanddate.com/sun/@8469718). Time points for Ramadan fasting are the call to prayer (Athan) for Fajr (abstinence or Imsak time, end of pre-fasting meal time or suhoor) and sunset or Maghrib (breakfast or Iftar meal time) prayer times. The sunrise prayer time is declared by Fajr Athan to be about mean of 80 min before the real sunrise time, as recorded in the Islamic calendar for prayer times. Therefore, the actual length of fasting time was calculated by adding 80 min to the time between the sunrise and sunset time points. Details of the pre-dawn Fajr and sunset Maghrib prayer time points on the Islamic calendar are available on the Islamic Finder website for Sharjah city, United Arab Emirates (https://www.islamicfinder.org/world/united-arab-emirates/292672/sharjah-prayer-times/). This showed that the length of fasting time for a specific day (time between the Fajr and Maghrib prayer times) was 787 min (approximately 13 h), which was close to the length of fasting time calculated using the solar calendar (sunrise and sunset time points).
Data synthesis and statistical analyses
Combined means were computed when studies included subgroups (e.g. normal body weight, overweight, obese), with different means and sd reported for each subgroup. P-values for the combined subgroup means were calculated. All descriptive and inferential tests were performed using Stata software (Stata, M.P., 15.0.: StataCorp, 2017).
We performed a series of one-group (pre-post) meta-analyses using a pre- and post-means model, sample size and P-values (paired groups). Hedges’ g was used to measure the effect size. Hedges’ g is an important measure of corrected effect size that is sensitive to even small samples (<20). An effect size is a quantitative measure of the magnitude of a change between two groups or one group under two conditions, for example, before and after. Details on Hedges’ g formula can be obtained from the original publication by Larry V. Hedges(Reference Hedges21). An effect size of ≤0·2 was considered a small effect, an effect size approximately 0·5 a medium effect and an effect size approximately 0·8 a large effect. A Hedges’ g value of one indicates the two groups differ by one sd, a g value of two indicates they differ by two sd, and so on. sd are equivalent to z-scores (1 sd = 1 z-score). In addition to Hedges’ g, we used forest plots to graphically present the results and illustrate point estimates for effect sizes and 95 % CI.
Random effects modelling was used for all analyses. By using random effects modelling, we therefore assume that there is not only one true effect size, rather a distribution of true effect sizes. We therefore wanted in this project to estimate the mean of this distribution of true effect sizes. I 2 statistics were used to assess the heterogeneity between included studies, and τ 2 statistics were used to assess heterogeneity within studies.
Comprehensive Meta-Analysis version 2(Reference Borenstein, Hedges and Higgins22) was used for all analyses. To confirm that our meta-analysis findings were not driven by a single study, we conducted leave-one-out sensitivity analyses (sensitivity analysis) by iteratively eliminating one study at a time. Moderator analysis was performed using subgroup analyses for categorical variables (country) and meta-regression for integer or decimal variables (age, percentage of male participants and fasting time per d).
Computing I 2 and τ 2 statistics were particularly important to examine heterogeneity due to explainable causes, for example, timing of data collection before Ramadan month and post-fasting.
The algorithm used to estimate a random effects meta-regression model was obtained using methods of moments(Reference Chen, Manning and Dupuis23). β-coefficients and P-values resulting from modelling were reported. Graphical plots were used to visually aid the interpretation of the results. Funnel-plot-based analysis was used to detect publication bias, and the nonparametric trim and fill method was used to confirm the findings(Reference Duval and Tweedie24). Finally, subgroup analysis for each MetS component was performed to investigate differences among countries. We performed this analysis if three or more studies were available from any given country.
Critical appraisal of studies (quality assessment)
Two reviewers (M. E. F. and A. A. O.) independently assessed the methodological quality of studies using a standardised checklist consisting of six items in terms of sample size and sampling technique, standardisation of data collection, appropriateness of statistical analyses, quality of reporting results and generalisability. The appraisal scores range between 0 and 6, with scores of 0–2 corresponds to low quality, 3–4 medium quality and 5–6 high quality. Quality score was set for each study by consensus after discussion (see online Supplementary Material 2).
Results
Eighty-five studies with a total of 4326 participants were included in this meta-analysis. Details of the sample size, participants’ sex and age, study design and major findings related to the MetS components for the included studies are shown in Table 1. All included studies used a pre-post design to report changes in the MetS components. Approximately 64 % of participants were male, and the median age was 31·5 years (range 15–80 years). The mean length of fasting during Ramadan across all studies was 828 (SD 90) min/d (range 668–1038 min).
Table 1. Characteristics and major findings of the included studies on the impact of Ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy people aged 15 years and above
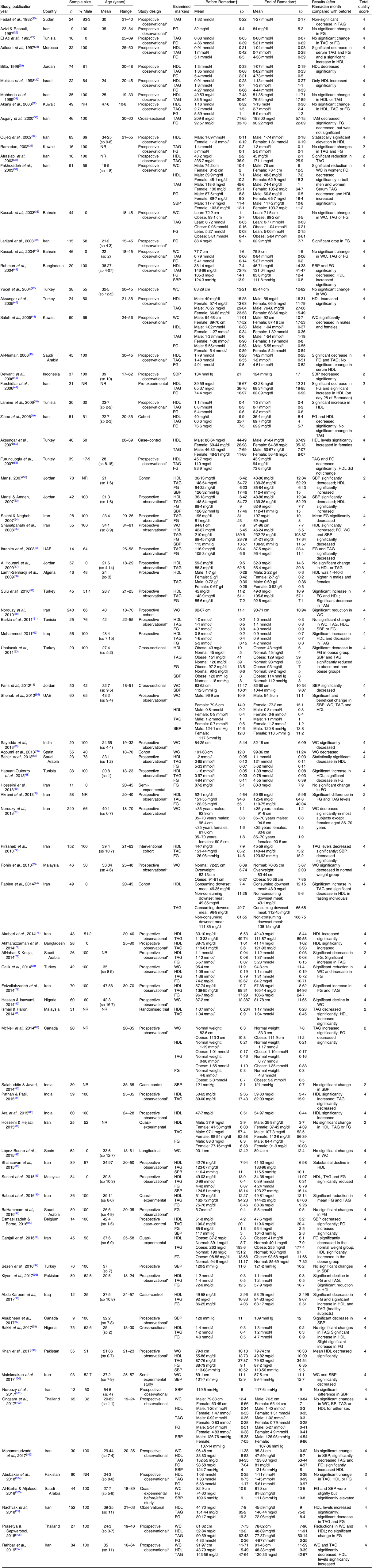
FG, fasting plasma/serum glucose; NR, not reported; SBP, systolic blood pressure; UAE, United Arab Emirates; WC, waist circumference.
* Not reported by the study authors.
† Results are transcribed from the original papers as reported. To convert from mg/dl to mmol/l, divide by 18. To convert mmol/l to mg/dl, multiply by 18.
Quality assessment of the analysed studies indicated that 15·3 % (13/85) were of low quality, while 84·7 % (72/85) were of medium quality.
Meta-analysis of the metabolic syndrome components
Table 2 summarises the number of studies (K), number of participants (N), participants’ mean age, percentage of male participants and fasting time (min/d). Visual inspection of the funnel plots indicated no bias in any of the included studies (online Supplementary Fig. S1–S5). Meta-analytic pooling WC, FG, TAG, HDL and SBP was performed, and the results expressed as K, N, Hedges’ g, 95 % CI and I 2: WC (K = 24, N 1557, Hedges’ g = −0·312, 95 % CI −0·387, −0·236, I 2 = 49 %; Fig. 2); FG (K = 51, N 2318, Hedges’ g = −0·101, 95 % CI −0·260, 0·004, I 2 = 26·6 %; Fig. 3); TAG (K = 63, N 2862, Hedges’ g = −0·088, 95 % CI −0·171, −0·004, I 2 = 78 %; Fig. 4); HDL (K = 57, N 2771, Hedges’ g = 0·150, 95 % CI 0·064, 0·236, I 2 = 79 %; Fig. 5) and SBP (K = 22, N 1172, Hedges’ g = −0·239, 95 % CI −0·372, −0·106, I 2 = 78 %; Fig. 6). Sensitivity analyses were performed for the MetS components by removing one study at a time to determine if the pooled effect size for each component was arbitrary or influenced by a single study.
Table 2. Characteristics and pooled analyses of included studies for each metabolic syndrome component
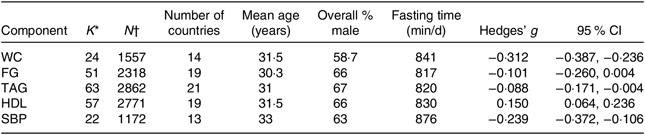
FG, fasting plasma glucose; SBP, systolic blood pressure; WC, waist circumference.
* K: denotes number of studies.
† N: denotes number of participants.
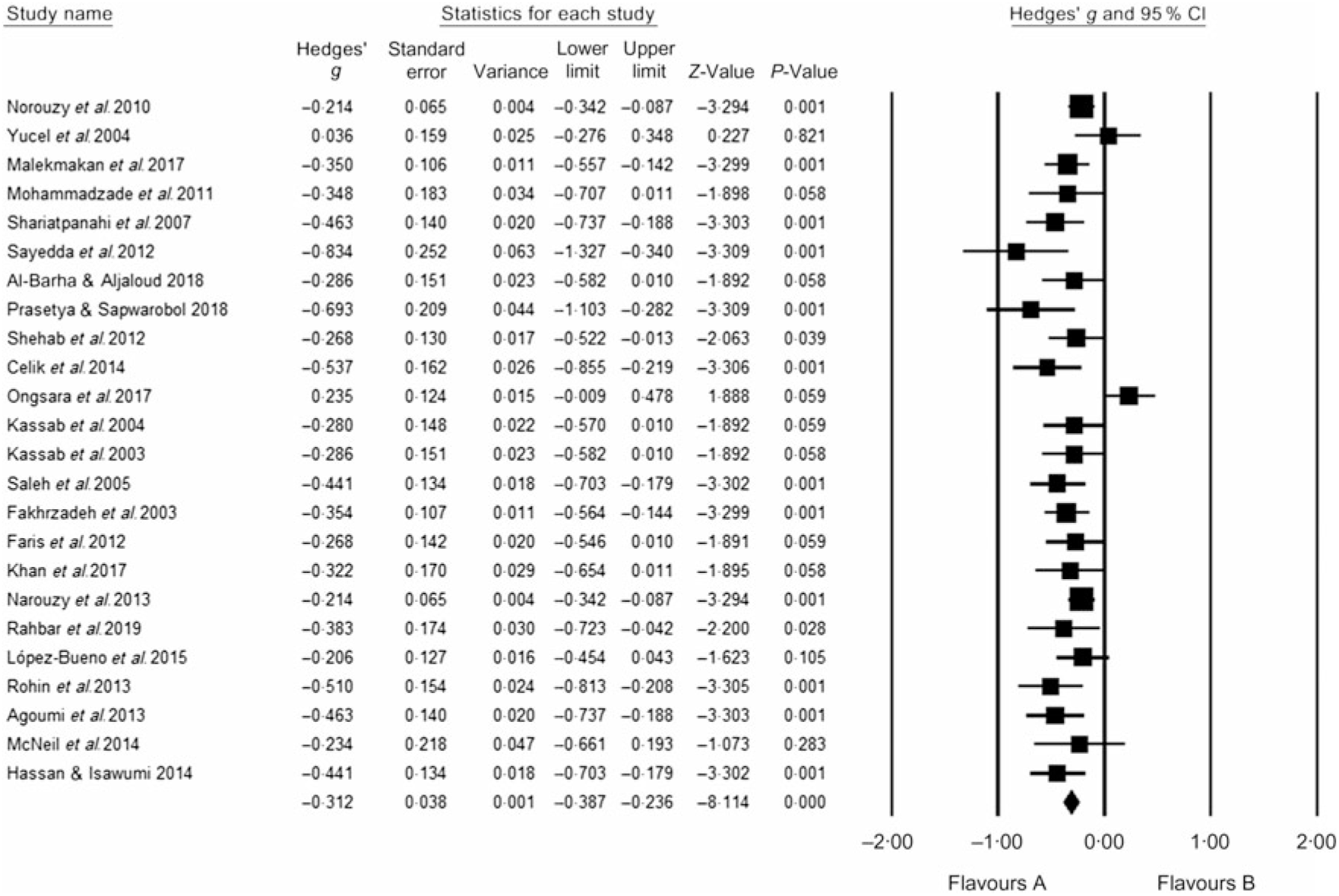
Fig. 2. According to Hedges’ g value with 95 % CI, small (−0·312) significant reduction in waist circumference was induced by Ramadan fasting. Heterogeneity statistics: 95 % CI −0·387, −0·236, I 2 = 49 %. Hedges’ g value is considered small when value = 0·2, medium = 0·5, large = 0·8.
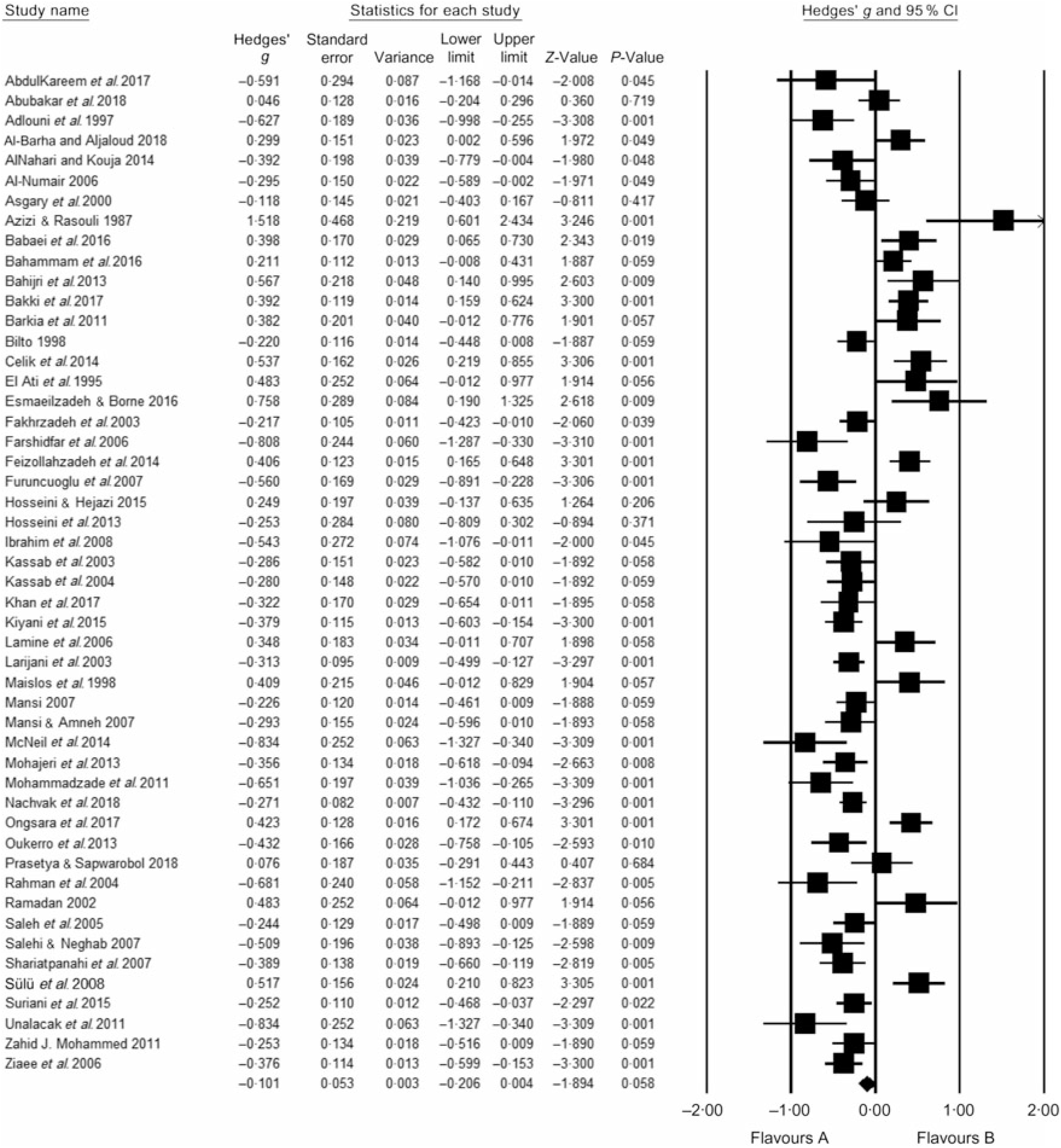
Fig. 3. According to Hedges’ g value with 95 % CI, small (−0·101) significant reduction in fasting glucose was induced by Ramadan fasting. Heterogeneity statistics: 95 % CI −0·206, 0·004, I 2 = 26·6 %. Hedges’ g value is considered small when value = 0·2, medium = 0·5, large = 0·8.
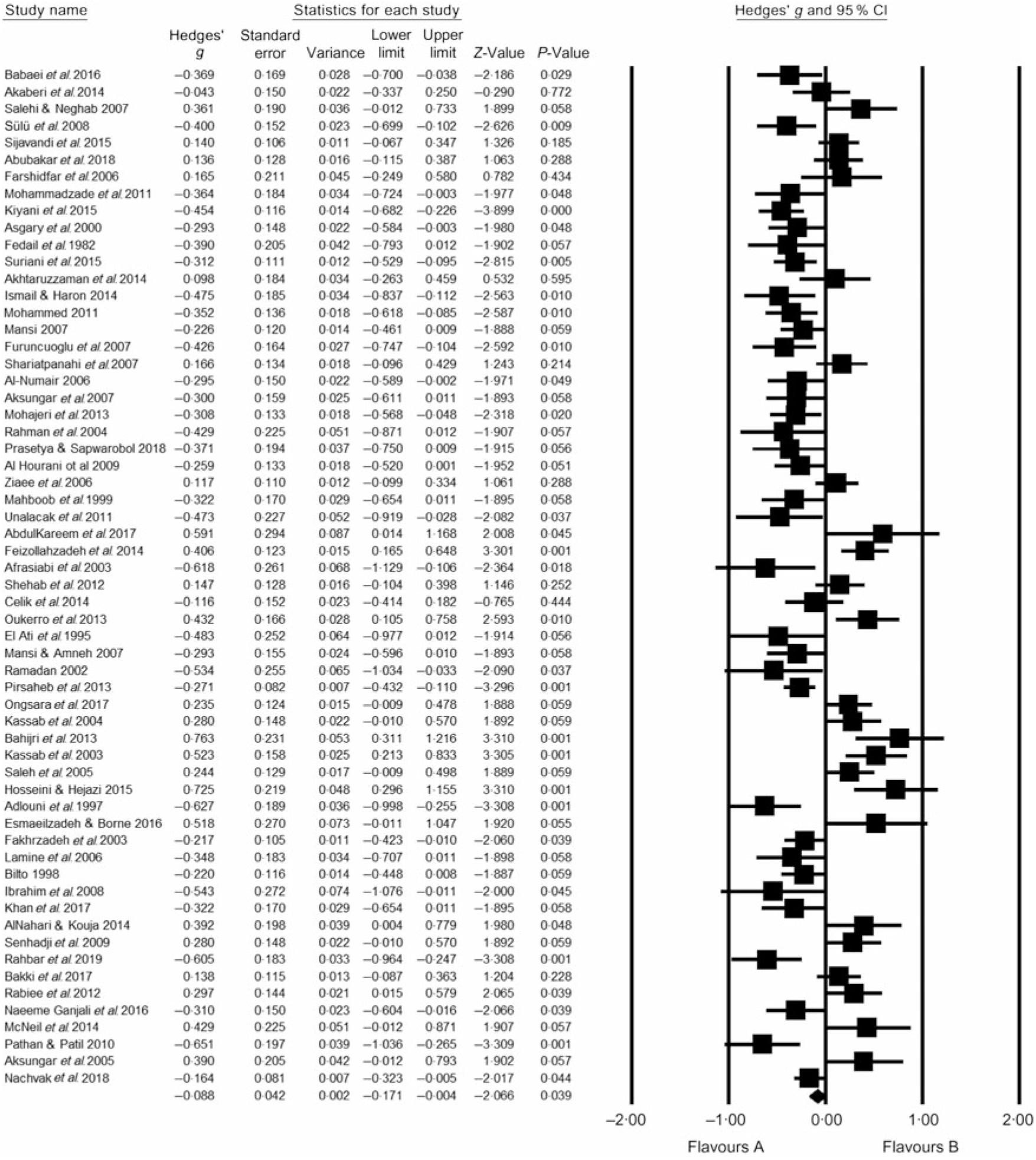
Fig. 4. According to Hedges’ g value with 95 % CI, small (−0·088) significant reduction in serum TAG was induced by Ramadan fasting. Heterogeneity statistics: 95 % CI −0·171, −0·004, I 2 = 78 %. Hedges’ g value is considered small when value = 0·2, medium = 0·5, large = 0·8.
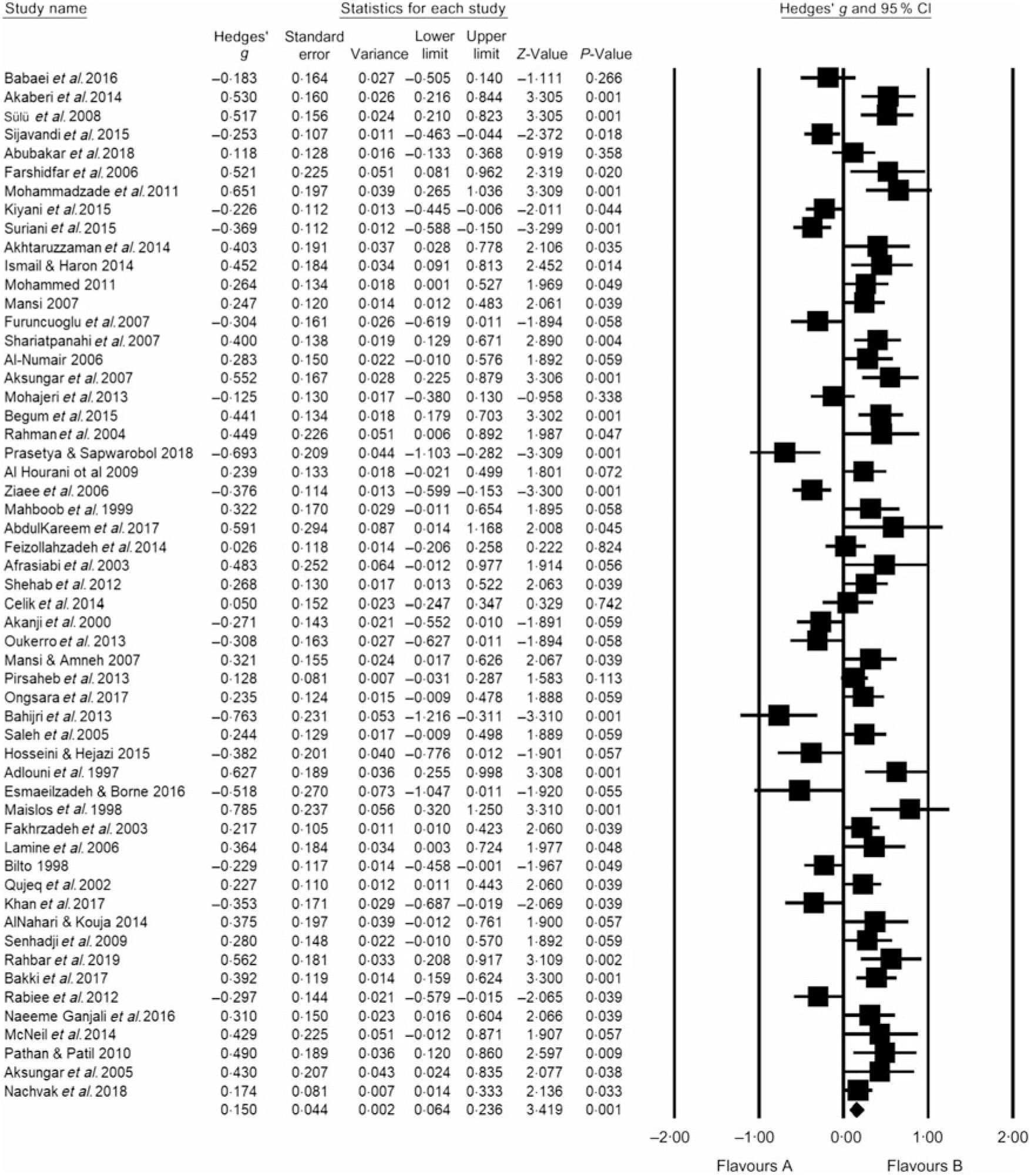
Fig. 5. According to Hedges’ g value with 95 % CI, small (0·150) significant increment in serum HDL-cholesterol was induced by Ramadan fasting. Heterogeneity statistics: 95 % CI 0·0640, 0·236, I 2 = 79 %. Hedges’ g value is considered small when value = 0·2, medium = 0·5, large = 0·8.
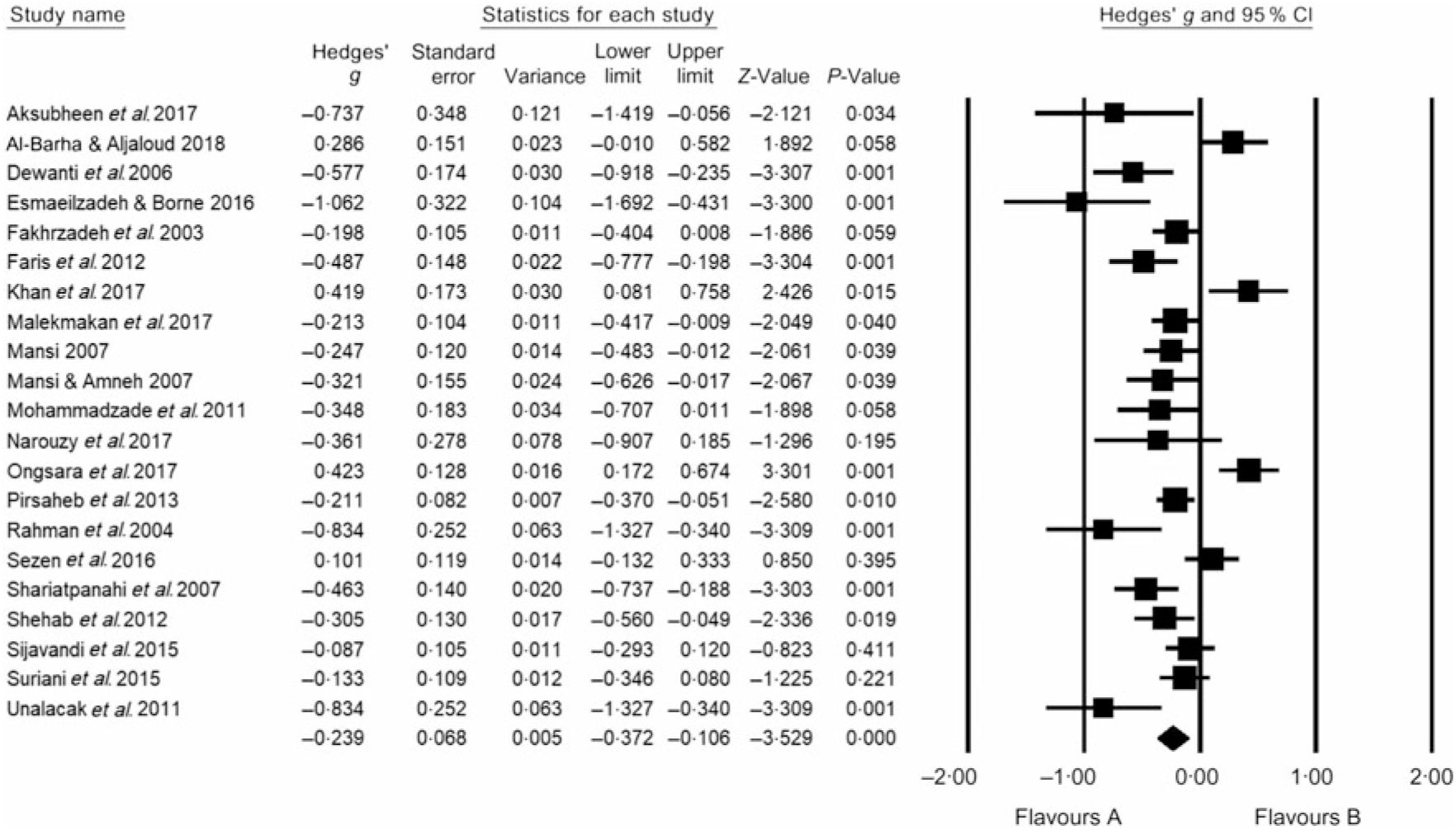
Fig. 6. According to Hedges’ g value with 95 % CI, small (−0·239) significant reduction in systolic blood pressure was induced by Ramadan fasting. Heterogeneity statistics: 95 % CI −0·372, −0·106, I 2 = 78 %. Hedges’ g value is considered small when value = 0·2, medium = 0·5, large = 0·8.
Moderator analysis for the metabolic syndrome components
Table 3 shows the results of the moderator analysis for each MetS component. We also performed subgroup analysis for specific MetS components for countries from which three or more studies were available to explore differences in findings among countries (Table 4).
Table 3. Overall Hedges’ g values for the metabolic syndrome components and statistical values for the three moderators (age, sex and fasting time) at the end of Ramadan
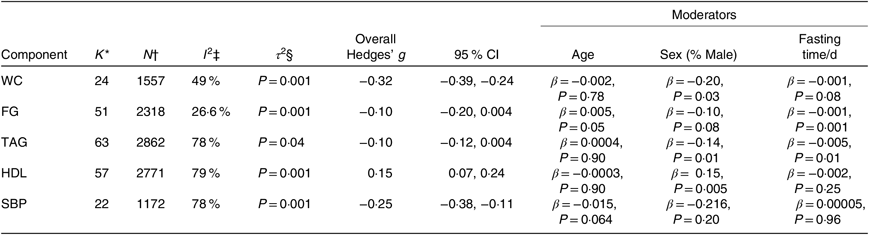
FG, fasting plasma glucose; SBP, systolic blood pressure; WC, waist circumference.
* K: denotes number of studies.
† N: denotes number of participants.
‡ I 2 statistic describes the percentage of variation across studies due to heterogeneity rather than chance(Reference Higgins, Thompson and Deeks108).
§ In a random-effects meta-analysis, the extent of variation among the effects observed in different studies (between-study variance) is referred to as τ 2(Reference Higgins, Thomas and Chandler109).
Table 4. Characteristics of studies included in each of the metabolic syndrome components reviewed and analysed by countries with three or more studies
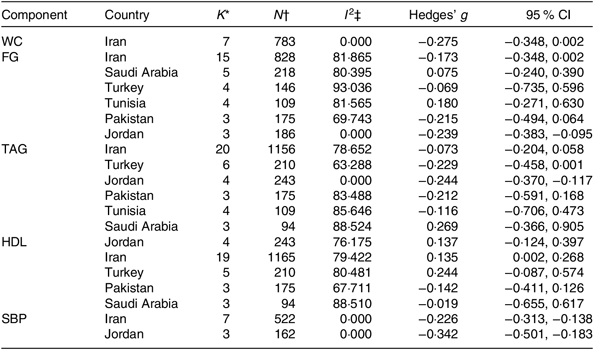
FG, fasting plasma glucose; SBP, systolic blood pressure; WC, waist circumference.
* K: denotes number of studies.
† N: denotes number of participants.
‡ I 2 statistic describes the percentage of variation across studies due to heterogeneity rather than chance(Reference Higgins, Thompson and Deeks108).
Waist circumference
Age (online Supplementary Fig. S6) and fasting time/d (online Supplementary Fig. S7) were NS moderators for changes in WC. However, sex was significant in explaining variation in WC (β = −0·20, P = 0·03) (online Supplementary Fig. S8), suggesting that women experienced a larger change in WC than men during RDIF. Only Iran contributed three or more studies that measured WC change during RDIF (K = 7, N 783): Hedges’ g = −0·275, 95 % CI −0·346, −0·204, I 2 = 0·0 %; online Supplementary Fig. S9).
Fasting glucose
Age (β = 0·005, P = 0·05; online Supplementary Fig. S10) and fasting time/d (β = −0·001, P = 0·001; online Supplementary Fig. S11) had significant impacts as moderators for changes in FG, whereas sex had no significant impact on FG changes during Ramadan fasting (online Supplementary Fig. S12). Six countries contributed three or more studies that measured FG changes during Ramadan month (online Supplementary Fig. S13): Iran (K = 15, N 828, Hedges’ g = −0·173, 95 % CI −0·348, 0·002, I 2 = 81·93 %), Saudi Arabia (K = 5, N 218, Hedges’ g = 0·075, 95 % CI −0·240, 0·390, I 2 = 80·41 %), Turkey (K = 4, N 146, Hedges’ g = −0·069, 95 % CI −0·735, 0·596, I 2 = 93·05 %), Tunisia (K = 4, N 109, Hedges’ g = 0·180, 95 % CI −0·271, 0·630, I 2 = 81·6 %), Jordan (K = 3, N 186, Hedges’ g = −0·239, 95 % CI −0·383, −0·095, I 2 = 0·0 %) and Pakistan (K = 3, N 175, Hedges’ g = −0·215, 95 % CI −0·494, 0·064, I 2 = 69·8 %).
TAG
Sex (β = −0·14, P = 0·01; online Supplementary Fig. S14) and fasting time/d (β = −0·005, P = 0·01; online Supplementary Fig. S15) had significant impacts as moderators for changes in TAG during RDIF, but age (online Supplementary Fig. S16) had no significant impact on TAG changes (Table 3). These findings suggested that women experienced a larger change in TAG than men during Ramadan month and that longer fasting time/d was associated with a greater reduction in TAG at the end of Ramadan. Six countries contributed three or more studies that measured TAG change during RDIF (online Supplementary Fig. S17): Iran (K = 20, N 1156, Hedges’ g = −0·073, 95 % CI −0·204, 0·058, I 2 = 78·7 %), Turkey (K = 6, N 210, Hedges’ g = −0·229, 95 % CI −0·458, 0·001, I 2 = 63·4 %), Jordan (K = 4, N 243, Hedges’ g = −0·244, 95 % CI −0·370, −0·117, I 2 = 0·0 %), Tunisia (K = 4, N 109, Hedges’ g = −0·116, 95 % CI −0·706, 0·473, I 2 85·6 %), Pakistan (K = 3, N 175, Hedges’ g = −0·212, 95 % CI −0·591, 0·168, I 2 = 83·5 %) and Saudi Arabia (K = 3, N 94, Hedges’ g = 0·269, 95 % CI −0·366, 0·905, I 2 = 88·5 %).
HDL-cholesterol
Age (online Supplementary Fig. S18) and fasting time/d (online Supplementary Fig. S19) were NS moderators for RDIF-induced changes in HDL. However, sex was significant in explaining the variation in HDL (β = 0·15, P = 0·005; online Supplementary Fig. S20), which suggested that men experienced larger changes in HDL than women during Ramadan month. Five countries contributed three or more studies that measured HDL changes during Ramadan month (online Supplementary Fig. S21): Iran (K = 19, N 1165, Hedges’ g = 0·135, 95 % CI 0·002, 0·268, I 2 = 79·4 %), Turkey (K = 6, N 210, Hedges’ g = 0·244, 95 % CI −0·087, 0·574, I 2 = 80·4 %), Jordan (K = 4, N 243, Hedges’ g = 0·137, 95 % CI −0·124, 0·397, I 2 = 76·1 %), Pakistan (K = 3, N 175, Hedges’ g = −0·142, 95 % CI −0·411, 0·126, I 2 = 67·6 %) and Saudi Arabia (K = 3, N 94, Hedges’ g = −0·019, 95 % CI −0·655, 0·617, I 2 = 88·5 %).
Systolic blood pressure
Age, sex and fasting time had no significant impact on SBP changes during RDIF (online Supplementary Figs. S22–S24). Two countries contributed three or more studies that measured SBP changes during RDIF (online Supplementary Fig. S25): Iran (K = 7, N 522, Hedges’ g = −0·226, 95 % CI −0·313, −0·138, I 2 = 0·0 %) and Jordan (K = 3, N 162, Hedges’ g = −0·342, 95 % CI −0·501, −0·183, I 2 = 0·0 %).
Discussion
This systematic review and meta-analysis was the first to clarify the impact of RDIF on the cluster of the MetS components. We found that RDIF incurred small significant improvements in the MetS components; namely, decreased WC, TAG, FG, and SBP and increased HDL.
It is worth to emphasise that subjects included in the present analysis were normal, not patients. We excluded those studies on patients during Ramadan month, as shown in the exclusion section and depicted in Fig. 1 of Preferred Reporting Items for Systematic Reviews and Meta-Analyses. It is well-known and commonly seen that elderly Muslim people are very keen to fast during Ramadan, even those who are patients and excused not to observe Ramadan fasting(Reference Azzoug, Mahgoun and Chentli110). Providing that the authors of the used articles did not mention that elderly people interrupted their fasting during Ramadan, we cannot assume that fasting days were reduced. Further, it is expected that differences may be existing in lipid profile changes between pre- and post-menopause women included in the present analysis, as supported by the published literature(Reference Reddy Kilim and Chandala111,Reference Fernandez and Murillo112) . However, such differences cannot be inferred from the present analysis and need to be executed in a special sub-group analysis.
The RDIF model is a widely known and well-studied model of religion-based intermittent fasting. Intermittent fasting is associated with improved human health(Reference Patterson, Laughlin and Lacroix113–Reference Trepanowski, Canale and Marshall116). The results of this meta-analysis expanded existing knowledge and confirmed that practicing RDIF had positive health impacts. RDIF had a beneficial effect on abdominal obesity, serum lipids, glucose metabolism and BP levels; all of which are the MetS components and risk factors for the development of insulin resistance, diabetes and CVD. The beneficial impact of RDIF on health is further reinforced by its ability to induce and activate antioxidant and anti-inflammatory mechanisms(Reference Faris, Jahrami and Obaideen8,Reference Faris, Kacimi and Al-Kurd18) .
The small reduction in WC shown in our results was consistent with (and may partially explain) the significant small RDIF-induced reduction in inflammatory markers including IL-6, TNF-α and hs-C-reactive protein and the oxidative stress marker malondialdehyde shown in a recent meta-analysis(Reference Faris, Jahrami and Obaideen8). The presence of the MetS has also been associated with lower plasma adiponectin levels(Reference Hajer, van der Graaf and Olijhoek117), indicating adipose tissue dysfunction and a two- to four-times increased risk for developing CVD and type 2 diabetes(Reference Isomaa, Almgren and Tuomi118). Similarly, several reports have indicated RDIF was associated with variable increments in adiponectin levels in fasting people(Reference Vardarli, Hammes and Vardarli119–Reference Mushtaq, Akram and Mushtaq121). Recently, we found that RDIF was associated with significant reduction in visceral fat surface area (measured by MRI) in fifty-seven overweight/obese participants, concomitant with significant reductions proinflammatory cytokines (IL-6 and TNF-α), and a significant increase in the anti-inflammatory cytokine IL-10(Reference Faris, Madkour and Obaideen122). In addition, Fernando et al. conducted a systematic review and meta-analysis on the impact of RDIF on body fatness. They found a significant reduction in fat percentage between pre- and post-Ramadan in people with overweight or obesity (−1·46, 95 % CI −2·57, −0·35 %, P = 0·010) compared with fasting subjects with normal weight(Reference Fernando, Zibellini and Harris9). This implied that RDIF incurs a pronounced protective effect against the MetS in people at risk for obesity.
In the present meta-analysis, there was a small but significant RDIF-induced reduction in serum TAG in healthy people, which may contribute to lowering the risk for atherogenesis. This plausible effect is supported by other reports that showed an anti-RDIF-induced atherogenic effect in ameliorating LDL-cholesterol and apo B, and improving anti-atherogenic apo A levels in subjects with normal weight(Reference Rahbar, Safavi and Rooholamini107,Reference Sarraf-Zadegan, Atashi and Naderi123,Reference Saleh, El-Kemery and Farrag124) and a small significant increase in HDL levels at the end of Ramadan. Other anti-atherogenic impacts for RDIF include: significant improvements in blood coagulation parameters(Reference Nahid, Sadegh and Amin125), along with significant reductions in total cholesterol(Reference Pathan and Patil84,Reference Ara, Jahan and Sultana85,Reference Kiyani, Memon and Amjad95,Reference Rahbar, Safavi and Rooholamini107) , very LDL(Reference Al Hourani, Atoum and Akel57,Reference Lamri-Senhadji, El Kebir and Belleville58,Reference Barkia, Mohamed and Smaoui61,Reference Nahid, Sadegh and Amin125) , LDL:HDL and cholesterol:HDL ratios(Reference Sarraf-Zadegan, Atashi and Naderi123,Reference Zare, Hajhashemi and Hassan126) and atherogenic index ((total cholesterol – HDL-cholesterol)/HDL-cholesterol)(Reference Aksungar, Eren and Ure43).
RDIF was also shown to improve endothelial function(Reference Yousefi, Faghfoori and Samadi127) by increasing nitric oxide production (important for normal endothelium)(Reference Daiber, Xia and Steven128) and improving the heat shock protein HSP70, which has been shown to possess atheroprotective and endothelial-improving effects(Reference Zare, Hajhashemi and Hassan126). Interestingly, the RDIF-induced cardioprotective effect extended for about 1 month after Ramadan month cessation(Reference El Ati, Beji and Danguir27,Reference Saleh, El-Kemery and Farrag124) . This indicated that RDIF has an annual short-term transient protective function against developing CVD. Finally, the small but significant reductions in the inflammatory (hs-C-reactive protein, TNF-α and IL-6) and oxidative stress (malondialdehyde) markers induced by RDIF reported in a recent meta-analysis(Reference Faris, Jahrami and Obaideen8) support the cardioprotective effect of Ramadan fasting. These markers have been shown to be involved in the etiopathogenesis of atherosclerosis and other CVD(Reference Soeki and Sata129–Reference Tangvarasittichai, Pongthaisong and Tangvarasittichai131). The potential for RDIF to improve antioxidants was supported by our recent findings that the relative gene expressions in obese subjects were significantly increased for three antioxidant genes (superoxide dismutase, SOD2; mitochondrial transcription factor A, TFAM; and nuclear factor erythroid 2-related factor 2, Nrf2) at the end of Ramadan, with percent increases of 90·5 %, 54·1 % and 411·5 % for these genes, respectively(Reference Madkour, El-Serafi and Jahrami132). However, the protective effect of RDIF against CVD does not appear to be substantiated by rigorous scientific evidence available to date. This suggests that more controlled research is warranted.
A decreased level of HDL-cholesterol is a basic component in the diagnosis and definition of the MetS(Reference Grundy2). In epidemiological studies, this abnormality has been closely associated with a higher risk for atherosclerotic CVD. Despite debate as to whether low HDL is involved in the pathogenesis of CVD, there is agreement that low HDL-cholesterol is a marker for increased risk for CVD(Reference Grundy2,Reference Grundy133) . In the present review, the presence of small but significant increments in HDL at the end of RDIF was an added RDIF-induced protective factor against CVD, as dyslipidemia and its cumulative metabolic derangements are all involved in the etiopathogenesis of the MetS and its complications.
Several mechanisms have been suggested to explain the relationship between hypertension and the MetS. These include the release of angiotensinogen from adipose tissue, expansion of intravascular volume, enhanced renal reabsorption of sodium (possibly due to insulin resistance), activations of the renin-angiotensin-aldosterone system and sympathetic nervous system and insulin resistance. Current consensus is that these factors act in conjunction to raise BP(Reference Grundy2). Consistent with the reduction in SBP, several reports indicated there was reduction in diastolic BP after RDIF(Reference Faris, Jahrami and Obaideen8,Reference Esmaeilzadeh and van de Borne92,Reference Malekmakan, Sayadi and Pakfetrat100,Reference Ongsara, Boonpol and Prompalad102) .
Insulin resistance with fatty acid flux is an accepted hypothesised mechanism for the underlying pathophysiology of the MetS, with low-grade chronic inflammation and oxidative stress being mechanisms that accompany the MetS(Reference McCracken, Monaghan and Sreenivasan134). A recent systematic review and meta-analysis revealed RDIF had a positive effect on lowering inflammatory and oxidative stress markers(Reference Faris, Jahrami and Obaideen8), with emphasis on IL-6 and TNF-α as the pro-inflammatory markers most involved in the pathogenesis of the MetS(Reference McCracken, Monaghan and Sreenivasan134). Several reports indicated RDIF was associated with variable changes in serum insulin and insulin resistance (presented as homeostatic model assessment of insulin resistance levels). Although several reports indicated a lack of significant changes in serum insulin and insulin resistance during RDIF(Reference Fakhrzadeh, Larijani and Sanjari37,Reference Celik, Saricicek and Saricicek78,Reference Radhakishun, Blokhuis and van Vliet135,Reference Nematy, Alinezhad-Namaghi and Rashed136) , other studies reported significant changes during RDIF(Reference Bahijri, Borai and Ajabnoor67–Reference Hosseini, Sardar and Hejazi69,Reference Prasetya and Sapwarobol106) . However, considerable variations in insulin and insulin resistance measurements imply that behavioural, dietary, and lifestyle factors affect these measurements, including quantity and quality of foods consumed, duration of fasting time, body weight and health status before fasting, differences in physical activity levels and differences in circadian rhythm and hormonal changes during RDIF.
The heterogeneity of the studies included in this meta-analysis regarding the MetS components could be attributed to various effects and confounding factors and perhaps explained by inconsistencies in the designs, procedures and interpretation of results of studies conducted during Ramadan. It is believed that a critical violation performed by many fasting people during Ramadan is skipping the predawn meal (suhoor), which may contribute to a significant daily energetic deficit and is likely to promote metabolic derangements, increased postprandial insulin levels and fat oxidation and induce confounding in the results(Reference Mansi and Amneh53).
Assuming that Ramadan fasting represents a form of time-restricted feeding as reported by Patterson & Sears(Reference Patterson and Sears114), our findings were consistent with other research on human and animal intermittent energy restriction and time-restricted feeding. There is a growing evidence base demonstrating short-to-medium term benefits of time-restricted feeding on glucose and lipid homeostasis, even in the absence of significant total daily energetic restriction (reduction in 25–40 % of total daily energetic intake). The majority of published research conducted during Ramadan revealed a lack of significant changes in total daily energetic intake during Ramadan compared with pre-fasting energetic intake(Reference Faris, Kacimi and Al-Kurd18,Reference Alsubheen, Ismail and Baker97,Reference Syam, Sobur and Abdullah137,Reference Harder-Lauridsen, Rosenberg and Benatti138) . By combining studies on RDIF, the present meta-analysis analysed a large sample, thereby increasing the power of the studies in showing the effects of RDIF. However, this meta-analysis had some limitations. In most included studies that evaluated the impact of RDIF on body weight, lipids and other metabolic profiles, there was no information as to whether participants consumed a predawn breakfast. During RDIF, subjects are at postprandial state if blood parameters are measured in the morning because of the predawn meal (suhoor). Several studies focused on RDIF did not report whether baseline laboratory parameters measured after an overnight fast before the initiation of Ramadan fasting were compared with laboratory parameters measured a couple of hours before the fast was broken at sunset, which would be the equivalent of an overnight fast. More importantly, the assessment of several key biomarkers of glucose and lipid metabolism and various hormones (including leptin, melatonin, ghrelin, cortisol and adiponectin) exhibits circadian rhythm and requires 24-h blood monitoring with multiple time points(Reference Gamble, Berry and Frank139). Measurement of these parameters at only one or a few time points may to lead to biased outcomes and potentially provide false data on increased/decreased levels, depending on the measurement time.
Dawn-to-sunset Ramadan fasting starts at dawn and ends at sunset. Therefore, fasting people have two major meals a day: breakfast before dawn and dinner after sunset, and may eat ad libitum from sunset until dawn. In general, it is a common practice for Ramadan fasters to work and fast during the daytime, have dinner, sleep and wake 1 h before dawn to eat and restart fasting at dawn. Most studies on RDIF did not provide explicit dietary information about the frequency of meals between sunset and dawn, meal content during night hours and sleeping times(Reference Alsubheen, Ismail and Baker97,Reference Elnakib140) . Furthermore, some study subjects may secretly break their fast and further confound the desired adaptive response. No information on compliance monitoring with fasting was available for any of the included studies. Well-designed clinical trials that evaluate the impact of dawn-to-sunset fasting on BMI and key metabolic parameters before, during and after fasting would provide important information about health maintenance(Reference Alsubheen, Ismail and Baker97).
Conclusions
It can be concluded from the reviewed and analysed the MetS components that RDIF showed small reductions in components associated with increased risk for and severity of the MetS (WC, SBP, TAG and FG), with a concomitant increase in anti-atherogenic HDL-cholesterol. These beneficial effects were also associated with variable improvements in other covariates involved in the etiopathogenesis factors, such as reduction in diastolic BP. The heterogeneity in the findings of the reviewed studies offers a picture of the varying dietary and lifestyle behaviours practiced during Ramadan month, along with variations in the duration of fasting and climatic and geographical conditions surrounding fasting people in different countries.
Ethical statement
This article does not contain any studies with human participants performed by any of the authors. For this type of study formal consent was not required
Acknowledgements
Authors express their thanks to Ms Faiza Kalam for assistance in article collection and data tabulation.
The present work did not receive any form of funding from any institution or funding body.
M. E. F. and H. A. J. contributed to the conception and design of the work. M. E. F. and A. A. O. participated in searching for and collecting articles. M. E. F., J. A. and A. A. O. participated in article reviews; J. A. principally participated in data extraction. H. A. J. performed all data analyses. M. E. F. and H. A. J. contributed to drafting the paper, critically revising the paper and providing intellectual contributions to strengthen the paper. All authors were involved in writing this paper and approved the final version for publication.
The authors have no conflicts of interest to declare.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S000711451900254X