Introduction
The black crested gibbon Nomascus concolor of China, northern Vietnam and north-western Laos is categorized as Critically Endangered on the IUCN Red List (IUCN, 2008). The greatest number, c. 1,000–1,300, occur in China, in several mountain areas (Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006). There are < 100 in northern Vietnam and an isolated population comprising 13 groups in the Nam Kan Valley, western Laos (Geissmann, Reference Geissmann2007a). The protection of N. concolor in China is thus of particular importance for the conservation of this species (Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006). In China the largest subpopulation of N. concolor, the subspecies N. c. jingdongensis, occurs on Wuliang Mountain, Yunnan, in southern China. A recent survey has shown that 98 groups inhabit Wuliang Mountain but that 22 of these live outside Wuliangshan Nature Reserve (Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006).
Gibbons are fastidious in their choice of habitats and therefore particularly sensitive to habitat degradation and fragmentation, and have been in dramatic decline for the last 30–40 years (Cheyne et al., Reference Cheyne, Thompson, Phillips, Hill and Limin2008). Habitat loss, degradation and fragmentation were noted as the major threats to nearly all gibbon species in the 2006 Asian Primate Red List workshop (Geissmann, Reference Geissmann2007b). Habitat deforestation and destruction has caused the population of N. concolor to shrink and become fragmented on Wuliang Mountain (Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006) and in Son La Province, Vietnam (Tri & Long, Reference Tri and Long2000).
A comprehensive understanding of the population biology and ecology of N. concolor is essential for the conservation of the species. However, except for simple descriptions of the forest type used by the species (Haimoff et al., Reference Haimoff, Yang, He and Chen1987; Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006), there have been no studies of how it uses its forest habitat, probably because of the typically rugged topography and the species' shyness of humans. Here we provide information on forest and strata use of one habituated N. concolor group. We describe the characteristics of forest types used by the group, assess the group's habitat preferences, and discuss the function of different forest types for the gibbons’ conservation.
Study area
The study was carried out between March 2005 and April 2006 at Dazhaizi on the border of Wuliangshan Nature Reserve, on the western slope of Wuliang Mountain, Yunnan (Fig. 1). The primary forest types are semi-humid evergreen broadleaved forests dominated by Castanopsis orthacanthus, Schima argentea, Cyclobalanopsis delavayi and Lithocarpus craibianus at 1,900–2,200 m altitude; mid mountain humid evergreen broadleaved forests dominated by Lithocarpus echinophorus, Lithocarpus hancei, Castanopsis wattii and Lithocarpus xylocarpus at 2,200–2,750 m, and rhododendron dwarf forests dominated by Rhododendron facetum and Rhododendron edgewothii at 2,800–3,000 m (Peng & Wu, Reference Peng and Wu1997). Alnus nepalensis forest is the main secondary forest type at 2,000–2,400 m (Peng & Wu, Reference Peng and Wu2001).

Fig. 1 The study area at Dazhaizi (grey rectangle) on the border of Wuliangshan Nature Reserve on Wuliang Mountain, Yunnan, China.
We identified forest types by dominant canopy species and canopy height, and combined semi-humid evergreen broadleaved forests and mid mountain humid evergreen broadleaved forests as primary forest because it is difficult to identify them in the field (Fan & Jiang, Reference Fan and Jiang2008a,Reference Fan and Jiangb). The predominant habitat types are primary forests at 1,900–2,700 m and rhododendron dwarf forests above 2,700 m. There were several large A. nepalensis secondary forest patches, mainly on slopes and ridges below 2,500 m. These forests were cleared for poppy plantation before the 1930s, and regenerated to secondary forests after 1958. From 1958 to 1986 the local people fired the forests nearly every year to prevent growth of young trees and promote herbs for grazing but the fire did not destroy the higher canopy. Near the forest is a large area of farmland abandoned 12–13 years ago that is covered mainly with Eupatorium adenophorum.
The lowest mean monthly temperature is in December (11.8°C) and the highest in June (19.6°C). Precipitation from April 2005 to March 2006 was 1,607 mm, with a wet season from May to October during which 96% of the rain fell (Fan & Jiang, Reference Fan and Jiang2008a,Reference Fan and Jiangb).
Methods
There were five groups of N. concolor living in this area. One group (G1) lived completely outside the Reserve and four (G2–G5) ranged both inside and outside the Reserve. In March 2003 we started to study G2 and G3. G3 was habituated by March 2005, and we then observed the group until April 2006. During the period of this study G3 comprised one adult male, two adult females (FY and FB), one subadult male, one juvenile male, one male infant born to FB in February 2004, and one infant born to FY in July 2005 (sex unknown).
In November 2004 we set 250 20 × 20 m plots at 100 m intervals over 2,100–2,600 m to cover nearly the whole known home range of G3 (Tian, Reference Tian2006). Of these, 52 plots were in secondary forest and the others in primary forest. No plots were placed in the rhododendron dwarf forest because at the time of placement the group had not been not observed there. In each plot we identified all trees with diameter at breast height (DBH) ≥ 10 cm to species and measured their DBH, height (with an optical range finder) and the height of the bottom branch. We also identified vine species. A full plant species list is provided in Tian (Reference Tian2006).
We followed G3 for 6–13 days each month from March 2005 to April 2006, totalling 125 days (845 h) of observation during the 14 months (Fan & Jiang, Reference Fan and Jiang2008b). Every 30 minutes we recorded the group's location and known landmarks with a global positioning system and marked these on a 1:6,250 topographical map. At each of these points we also recorded forest type, individual activity and height above ground for each visible individual. Activity was classed as resting, travelling, feeding, calling, playing and other (Fan et al., Reference Fan, Ni, Sun, Huang and Jiang2008). When the group ranged on cliffs we recorded the location but not behaviour.
We examined G3's forest preference by superimposing a 100 × 100 m (i.e. 1 ha) grid on the range maps, which were overlaid with the map of forest types. If a grid cell contained > 1 forest type, we noted the predominant type as the forest type in that grid cell. The group's home range was 151 ha during the study period. There was primary forest in 118 grids, secondary forest in 31 and rhododendron dwarf forest in two (Fan & Jiang, Reference Fan and Jiang2008a,Reference Fan and Jiangb).
Overall and monthly habitat electivity indices (EI; Wallace, Reference Wallace2006) were calculated for the three forest types. Index values varied from -1 (not selected) to +1 (highly selected):
where ri is the percentage of habitat use i in the sample and ni is the relative availability of habitat i in the gibbon home range.
We assigned tree height and height of gibbons in the trees to six categories: 0–5, 6–10, 11–15, 16–20, 21–25, >25 m. We performed all statistical analyses using SPSS v. 11 (SPSS, Chicago, USA). We first tested the data on forest characteristics for normality with the Kolmogorov-Smirnov test and then, because they were non-normal, we used the Mann-Whitney U test for differences between these characteristics for different forest types. We used χ2 to test whether gibbons used different forest types randomly and used forest strata randomly in different forest types.
Results
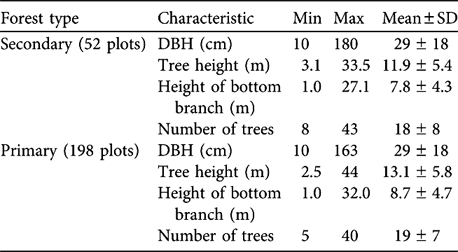
There was no difference in tree density or DBH between primary and secondary forest (Table 2; density: Z = -1.100, df = 1, P = 0.271; DBH: Z = -1.298, df = 1, P = 0.194). The distribution of tree height differed significantly between primary forest and secondary forest (χ2 = 35.013, df = 5, P < 0.001). Secondary forest had more trees of height ≤ 10 m, and primary forest more of height >10 m (Table 1). Tree height and bottom branch height in the primary forest was significantly higher than in the secondary forest (Mann Whitney; tree height: Z = -6.094, df = 1, P = 0.000; bottom branch height: Z = -5.186, df = 1, P = 0.000).
Table 1 Percentages of trees in six height classes in primary, secondary and dwarf forest, and percentage usage of each by gibbons, in Dazhaizi (Fig. 1). Of a total of 4,864 observations 4,531, 312 and 21 were in primary, secondary and dwarf forest, respectively.
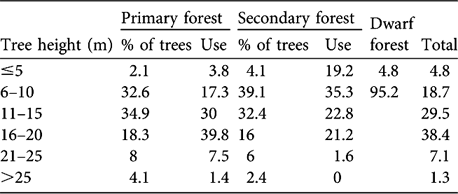
Table 2 Characteristics of secondary and primary forest in Dazhaizi (Fig. 1).
Although we observed gibbons to eat 77 plant species, 10 accounted for 77% of the diet (Fan et al., Reference Fan, Ni, Sun, Huang and Jiang2009). Six (Ficus neriifolia, Ficus sarmentosa, Saurauia napaulensis, Photinia serrulatawere, Kadsura interior and Pentapanax leschenaultia) of these were found only in the primary forest, Leucosceptrum canum only in the secondary forest, and three (Betula alnoides, Pholidota articulata and Tetrastigma delavagi) in both primary and secondary forest. We defined important food patches as those in which three or more individuals fed on the same food species for > 10 minutes (Fan & Jiang, Reference Fan and Jiang2008a,Reference Fan and Jiangb). We recorded 132 such food patches and they were not distributed in the three different forest types (89, 11 and 0% in primary, secondary and dwarf forest respectively) with an expected frequency equal to the area of each forest type (χ2 = 8.511, df = 1, P = 0.014).
We recorded G3 at 1,821 locations. They spent 92.9% (1,692 records) of their time in the primary forest, 6.6% (120) in secondary forest, and 0.5% (9) in dwarf forest. They never used the E. adenophorum grassland. The overall and monthly habitat electivity indices are given in Fig. 2. The study group usually preferred primary forest but they selected secondary forest in February 2006 (Fig. 2), when few fruits were available but L. canum was blossoming. During L. canum flowering gibbons intensively consumed the flowers, often visiting the trees 2–3 times per day. The mean height of these trees was 7.7 ± SD 2.3 m (range 3.9–14.5 m, n = 125). Gibbons only occasionally used the dwarf forest, in 5 months of the 14-month study (Fig. 2). They never stayed long there but simply traversed it to go to the primary forest. The boundary of the dwarf forest usually constituted the upper limit of the gibbon home range.

Fig. 2 Overall and monthly habitat electivity indices (see text for details) for the black crested gibbon from March 2005 to April 2006.
There was a significant difference in the time budget of the gibbons in the different forest types (χ2 = 92.253, P < 0.001). They spent more time in feeding related behaviour (feeding and travelling) and less time resting when they ranged in secondary forest, and spent more time travelling and less time resting when in the dwarf forest (Table 3).
Table 3 The activity budget of gibbons in primary, secondary and dwarf forest in Dazhiazi (Fig. 1). Of a total of 6,347 observations 5,945, 372 and 30 were in primary, secondary and dwarf forest, respectively.

Gibbons did not use the forest strata randomly when they ranged in either primary (χ2 = 3243.667, df = 5, P < 0.001) or secondary forest (χ2 = 90.596, df = 4, P < 0.001; Table 1), and there was a significant difference in their strata use in different forest types (χ2 = 285.395, df = 5, P = 0.000). They concentrated their activities (69.8%) in the middle layer (11–20 m) in primary forest but spent more time in the lower layer (≤ 10 m; 54.5%) in secondary forest, where they fed on the flowers of the small L. canum trees. Although trees in the dwarf forest were usually < 8 m tall, the gibbons usually used the upper stratum of the trees when they ranged there (Table 1).
Discussion
Primary forest provided most of the food resources for the studied group of N. concolor. Except for L. canum, the other nine important food species were in the primary forest and 89% of the patches where the group fed occurred there. Although the secondary forest in the study area has been regenerating for > 50 years, the plant species diversity is lower than that in the primary forest. Six of the 10 preferred food plant species could not be found in the secondary forest, which suggests that these species are late-succession species. Unfortunately, the local people living near Wuliangshan Nature Reserve still continue to clear the primary forest outside the Reserve to plant tea and nut trees and to halt growth of secondary forest. Moreover, there has been some selective logging outside the Reserve focused on B. alnoides, one of the 10 preferred food species. A study in the Sungai Tekam area of the Malay Peninsula has shown that if only 4% of trees are extracted, 45% of the total stand is damaged during access, felling and extraction (Chivers, Reference Chivers, Caldecott and Miles2005). Thus selective logging threatens the gibbons living outside the Reserve. In 2005 group G1 lived outside the Reserve and disappeared after selective logging.
Besides food supply, primary forests also provided sleeping and singing trees and other important resources for the gibbons. All the sleeping trees for the group members (Fan & Jiang, Reference Fan and Jiang2008a), as well as the singing trees of the adult male (Authors, unpubl. data), were in primary forest, which has a well connected canopy that provides cover and suitable locomotion supports.
The group made use of food resources in secondary forest in every month except July, and preferred this forest in February when few fruits were available within their range but L. canum was blossoming. Secondary forest trees are in two layers. The first is 18–25 m in height and contains as its primary species A. nepalensis, which has a sparse crown and large gaps between crowns (Tian, Reference Tian2006). These characteristics make it difficult for gibbons to move fast and make them easy to detect. Although the clouded leopard Neofelis nebulosa is a potential predator of N. concolor on Wuliang Mountain (Jiang et al., Reference Jiang, Ma, Wang, Sheeran and Poirier1994) people are probably the main predator of gibbons in this area. Almost all local hunters reported killing gibbons, with c. 30 killing a total of 105 gibbons from the 1950s to the 1980s (Jiang et al., Reference Jiang, Luo, Zhao, Li and Liu2006). Without human predators, secondary forest could serve as a corridor between primary forest patches, especially for dispersing subadults.
The study group spent only 0.5% of their time in the dwarf forest. It provided few food resources, was < 8 m in height, and the dense branches of the Rhododendron spp. there are not suitable for gibbons’ rapid suspended movement. Although they tended to travel in the upper stratum, they were thus under increased risk of attack by predators. Dwarf forest is presently the dominate forest type near the ridge of Wuliang Moutain. If the population were to communicate with the population on the other side of the ridge, they would need to travel across the dwarf forest. Whether or not gibbons can cross this forest is unclear because of the difficulty in following them there. If they can do so, this could improve the gene pool of this small population and thus increase chances for long-term survival.
Primary forest on Wuliang Mountain appears to be critical for the survival of N. concolor. It provides them with food resources, sleeping and singing trees, cover and suitable locomotory supports. Selective logging and agriculture encroachment in the primary forest needs to be halted even outside Wuliangshan Nature Reserve.
Compared with E. adenophorum grassland within the study site, secondary forest not only provides corridors for dispersing gibbons but also important food resources in some months. To enhance the gibbons’ habitat, the grassland needs to be restored to forest. In early successional stages few tree species, except A. nepalensis, Myrica esculenta, B. alnoides and L. canum, have survived in the E. adenophorum grassland (Authors, pers. obs.). These are the pioneer species in succession and are thus good candidates for reforestation. A. nepalensis and B. alnoides grow fast, with some individuals of A. nepalensis reaching a height of c. 10 m 12 years after the farmland was abandoned. These two fast growing species can provide closed canopy for other late-succession species 20–30 years later (pers. obs.). M. esculenta, B. alnoides and L. canum can provide food for gibbons between February and April.
The dwarf forest near the ridge and far from villages and little disturbed by people faces the threat of fire because it is dry. Its protection also needs attention because it has the potential to provide corridors for gibbons to cross the ridge.
Our conclusions and conservation recommendations were provided to the local government and Management Bureau of Wuliangshan Nature Reserve (MBWNR). The gibbon's habitat outside the Reserve is now being protected by the local government, and MBWNR are planning to restore the land currently occupied by E. adenophorum grassland. We are, meanwhile, continuing our research on N. conolcor. Finally, black crested gibbons on other mountains in Yunnan live in a similar habitat (semi-humid evergreen broadleaved forests and mid mountain humid evergreen broadleaved forests). Our recommendations may also be applicable to the recovery of gibbon habitat in these areas.
Acknowledgements
This study was carried out at the Black Crested Gibbon Monitoring Station on Wuliang Mountain and was supported by the National Natural Science Foundation of China (# 30670270), and by the Knowledge Innovation Programme of the Chinese Academy of Sciences (#KSCX2-SW-119) and doctoral funding from Dali University (KY430840). We acknowledge our field assistants Liu Yekun and Liu Yeyong for their kind help.
Biographical sketches
Fan Peng-Fei has studied the behavioural ecology and conservation biology of gibbons in Yunnan and is now studying the ecology and behaviour of Cao Vit gibbons in Guangxi. Jiang Xue-Long studies the behavioural ecology, conservation biology and mating system of the black crested gibbon in Yunnan. Tian Chang-Cheng specializes in the study of plant diversity.







