Introduction
The discovery and description of the conodont animal from the Carboniferous of Scotland (Briggs et al., Reference Briggs, Clarkson and Aldridge1983) inevitably stimulated discussion concerning the relationship of conodonts to early vertebrates (Aldridge et al., Reference Aldridge, Briggs, Clarkson and Smith1986, Reference Aldridge, Smith, Clarkson and Clark1993; Kemp and Nichol, Reference Kemp and Nicoll1995; Donoghue et al., Reference Donoghue, Purnell and Aldridge1998, Reference Donoghue, Forey and Aldridge2000; Blieck et al., Reference Blieck, Burrow, Schultze, Rexroad, Bultynck and Nowlan2010; Turner et al., Reference Turner, Burrow, Schultze, Blieck, Reif, Rexroad, Bultynck and Nowlan2010; Donoghue and Keating, Reference Donoghue and Keating2014). In these deliberations, the main focus of discussion was on euconodonts, the most familiar of the three descriptive groups (protoconodonts, paraconodonts, euconodonts) recognized on the basis of mainly Cambrian material by Bengtson (Reference Bengtson1983). The affinity of protoconodonts with chaetognaths rather than vertebrates was established by Szaniawski (Reference Szaniawski1982, Reference Szaniawski1983, Reference Szaniawski1987, Reference Szaniawski2002; Vannier et al., Reference Vannier, Steiner, Renvoisé, Hu and Casanona2007), but Bengtson’s (Reference Bengtson1983) suggestion that euconodonts were derived from paraconodonts ultimately formed the focus for the investigation of conodont element structure between these latter two groups by Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013, Reference Murdock, Rayfield and Donoghue2014). Similarity between the crown tissue of euconodont elements and vertebrate enamel was attributed to convergence rather than homology (Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013, Reference Murdock, Rayfield and Donoghue2014), although the vertebrate affinity of conodonts was not questioned. Donoghue and Keating (Reference Donoghue and Keating2014) accepted the conclusions of Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013) while maintaining assignment of conodonts to the vertebrates on the basis of their soft part anatomy (Aldridge et al., Reference Aldridge, Smith, Clarkson and Clark1993; Pridmore et al., Reference Pridmore, Banvick and Nicoll1997), despite the objections of Blieck et al. (Reference Blieck, Burrow, Schultze, Rexroad, Bultynck and Nowlan2010) and Turner et al. (Reference Turner, Burrow, Schultze, Blieck, Reif, Rexroad, Bultynck and Nowlan2010).
Microscopic, coiled, phosphatic early Cambrian sclerites described herein show little overall morphological similarity to the diverse and widely distributed conodont elements recovered from Cambrian–Triassic sediments. Their extraordinary mode of growth indicates, however, that they can be compared to some Cambrian (Series 3–4) paraconodonts, and this observation is developed to promote a tentative extension of the model of early vertebrate (conodont) evolution proposed by Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013) back into the early Cambrian (Cambrian Series 2).
The Cambrian sclerites are assigned to Tarimspira Yue and Gao, Reference Yue and Gao1992. The type species, Tarimspira plana Yue and Gao, Reference Yue and Gao1992, was originally described from the lower Cambrian (Cambrian Series 2) of western China and is unusual because of its tightly coiled, laterally compressed, planispiral form (Fig. 1). Yue and Gao (Reference Yue and Gao1992) compared its shape with that of bellerophontiform mollusks, and there is a seductive resemblance to isostrophically coiled shells of macromolluskan genera such as Joleaudella Patte, Reference Patte1929. However, leaving aside the great difference in size, geological age, and phosphatic composition, examination of the method of shell accretion of Tarimspira quickly demonstrates that the similarity is superficial, as recognized by Yue and Gao (Reference Yue and Gao1992), merely reflecting their common logarithmic growth. Tarimspira plana is a relatively solid sclerite with externally deposited growth lamellae that extend from the base to envelop the lateral surfaces. While this method of accretion is also seen in the early growth stages of paraconodonts, the latter rapidly develop a conical form and a deep basal cavity during ontogeny that is not present in Tarimspira (Müller, Reference Müller1959; Andres, Reference Andres1988; Müller and Hinz, Reference Müller and Hinz1991; Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013).
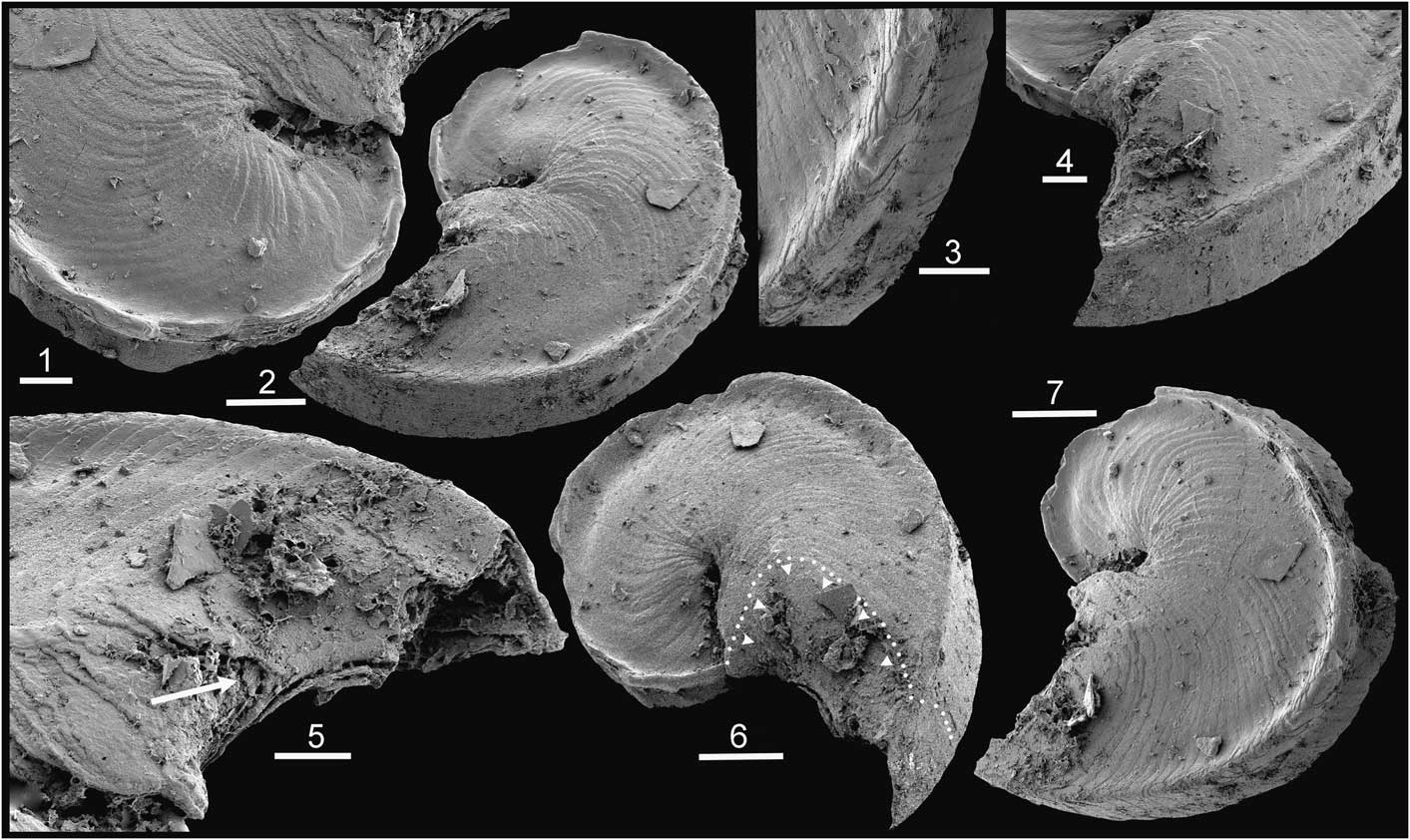
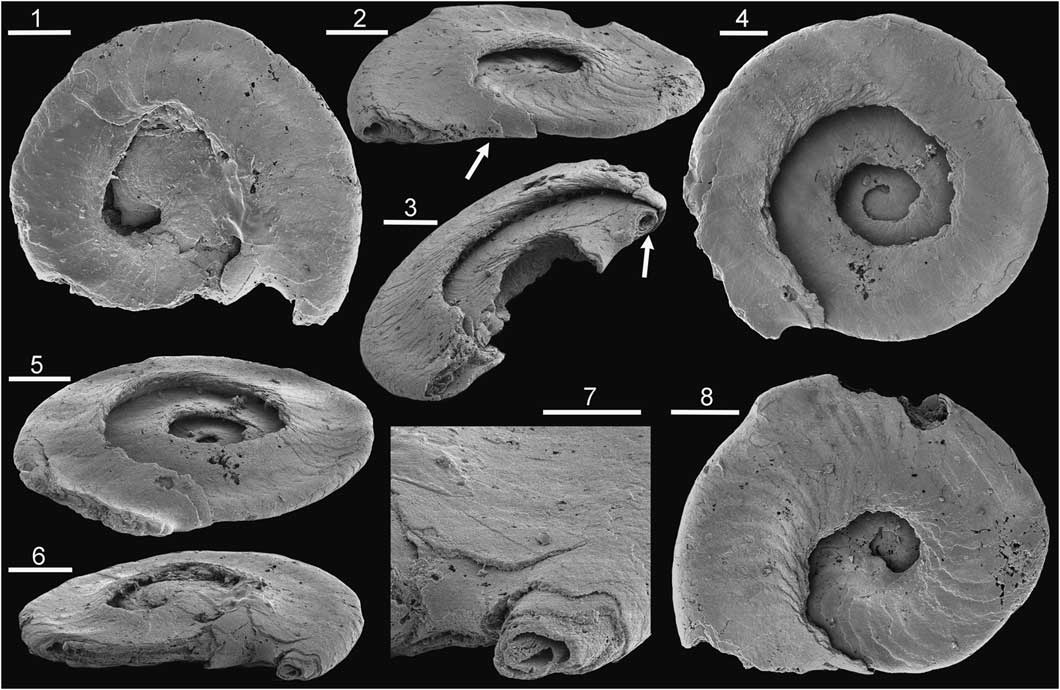
Figure 1 Tarimspira plana Yue and Gao, Reference Yue and Gao1992, PMU 31847 from GGU sample 255522, Aftenstjernesø Formation (Cambrian Series 2, Stage 4), Løndal, Peary Land, North Greenland. (1) Detail of umbilical region and growth lamellae; (2, 7) oblique lateral views showing peripheral keels; (3) detail of peripheral keels; (4, 5) latest growth stage showing last deposited growth lamella extending from base onto lateral areas (arrow in 5); (6) slightly oblique lateral showing extent of last deposited growth lamella and its direction of slope (arrows). (1, 3–5) Scale bars = 50 µm; (2, 6, 7) scale bars = 100 µm.
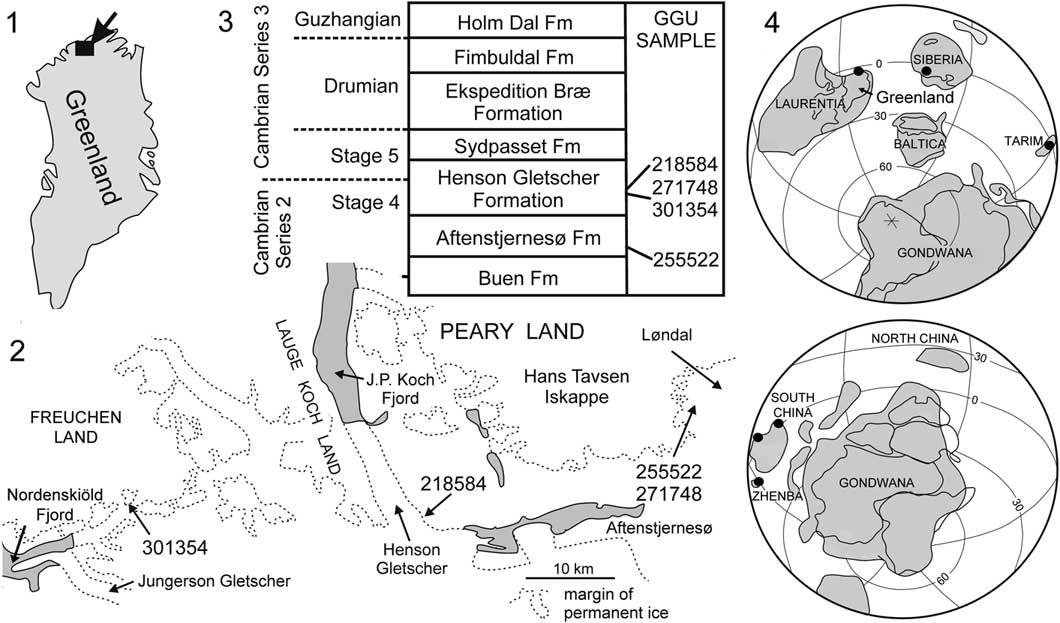
Tarimspira is documented herein from several horizons within the lower Cambrian (Cambrian Series 2, Stage 4) of North Greenland (Fig. 2), where it is represented by Tarimspira artemi n. sp. and a single specimen of the type species, Tarimspira plana. The occurrences represent its first description from Laurentia, consolidating a brief identification (as Fengzuella) by Kouchinsky et al. (Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015, p. 481). Tarimspira has previously been described from Siberia (Kouchinsky et al., Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015) and several terranes in China (Yue and Gao, Reference Yue and Gao1992; Steiner et al., Reference Steiner, Li, Qian, Zhu and Erdtman2003; Yang et al., Reference Yang, Steiner and Kepp2015). Although few, these currently known occurrences display a clear equatorial distribution (Fig. 2.4).
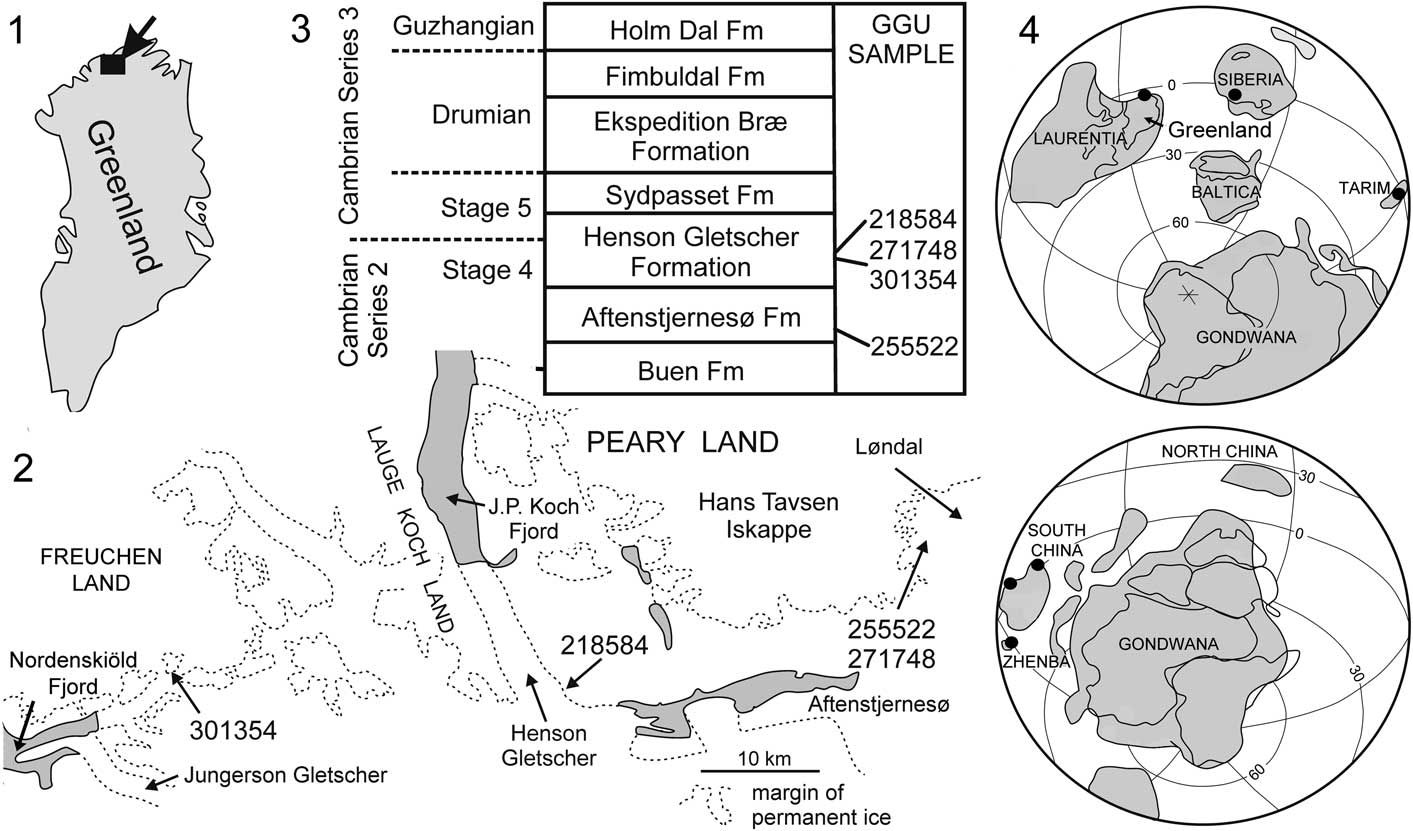
Figure 2 (1, 2) Collection localities of GGU samples from North Greenland yielding Tarimspira; (3) Cambrian stratigraphy in the Freuchen Land–Peary Land region with derivation of samples; (4) distribution of Tarimspira during Cambrian Series 2.
Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) commented that Chinese specimens occurred in marginal shelf environments. This pattern is confirmed by the present records from shelf deposits of the Aftenstjernesø and Henson Gletscher formations of North Greenland (Ineson and Peel, Reference Ineson and Peel1997; Fig. 2.3).
Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) recognized that the co-occurrence of Tarimspira (as Fengzuella) zhejiangensis (He and Yu, Reference He and Yu1992) with two other sclerite morphotypes in samples from South China indicated that it formed part of the scleritome of an undetermined organism. This deduction is confirmed herein with the description of a new species, Tarimspira artemi, from the Henson Gletscher Formation of North Greenland in which two sclerite morphotypes are known currently from the scleritome. Tarimspira artemi n. sp. is also recorded from the Emyaksin Formation (Cambrian Series 2, Stage 3) of northern Siberia, where it was described under the name Fengzuella zhejiangensis by Kouchinsky et al. (Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015). Tarimspira plana, originally described from the Tarim terrane of western China (Yue and Gao, Reference Yue and Gao1992), is now described from the basal Aftenstjernesø Formation of Peary Land, North Greenland (Figs. 1, 2); this species also appears to contain at least two sclerite morphotypes in its scleritome.
Geological background and materials
The geological evolution and lithostratigraphy of the Cambrian of North Greenland were described by Higgins et al. (Reference Higgins, Ineson, Peel, Surlyk and Sønderholm1991) and Ineson and Peel (Reference Ineson and Peel1997, Reference Ineson and Peel2011). Siliciclastic shelf sediments of the Buen Formation (Cambrian Series 2, Stages 3–4; Fig. 2.3) are overlain by carbonates (Aftenstjernesø Formation) that represent the initial stage in the establishment of a major carbonate platform that extended east–west across North Greenland during the Cambrian–Silurian. In the Freuchen Land and Peary Land region (Fig. 2.2), the Cambrian carbonates form a northward prograding complex in which recessive units of dark carbonates and shales (Henson Gletscher, Ekspedition Bræ, and Holm Dal formations; Fig. 2.3), representing periods of relative lowstand of sea level, are separated by carbonate turbidites and mass flow deposits (Aftenstjernesø, Sydpasset, and Fimbuldal formations; Fig. 2.3) deposited during periods of sea level highstand (Ineson and Peel, Reference Ineson and Peel1997).
Material of Tarimspira from North Greenland was collected from the Aftenstjernesø and Henson Gletscher formations (early Cambrian; Cambrian Series 2, Stage 4; Fig. 2.3). In Peary Land and Freuchen Land, the Aftenstjernesø Formation yields fossils only from its basal member, a widespread, sediment-starved, outer ramp sequence in which hardgrounds are conspicuous (Frykman, Reference Frykman1980; Ineson and Peel, Reference Ineson and Peel1997; Peel, Reference Peel2017). The member is 3–7 m thick and is mainly composed of glauconitic and phosphatic dolostones and limestones that vary in texture from massive to laminated and burrowed.
The Henson Gletscher Formation is dominated by thinly bedded, sooty black limestones, dolostones, and shales that were deposited in an off-platform setting (Higgins et al., Reference Higgins, Ineson, Peel, Surlyk and Sønderholm1991; Ineson and Peel, Reference Ineson and Peel1997; Geyer and Peel, Reference Geyer and Peel2011). In most sections, a lower dark recessive member is overlain by a prominent median unit of pale sandstones and a recessive upper dark member. Diverse faunas (Cambrian Series 2, Stage 4) from the Henson Gletscher Formation were monographed by Blaker and Peel (Reference Blaker and Peel1997), Geyer and Peel (Reference Geyer and Peel2011), and Peel et al. (Reference Peel, Streng, Geyer, Kouchinsky and Skovsted2016). Trilobites of Cambrian Series 3 age were described by Robison (Reference Robison1984) and Babcock (Reference Babcock1994a, Reference Babcockb).
Samples were collected during regional mapping campaigns (1978–1980; 1984–1985) of the Geological Survey of Greenland (Peel and Sønderholm, Reference Peel and Sønderholm1991). Specimens were etched from the limestone with 10% acetic acid, hand picked from sieved fractions (250 µm and coarser), and gold-coated prior to scanning electron microscopy. Images were assembled in Adobe Photoshop CS4.
Locality information
GGU sample 255522 was collected by P. Frykman on 14 July 1979 from limestone within the lowest 1 m of the basal member of the Aftenstjernesø Formation in Løndal, Peary Land (82°17.5'N, 37°03'W; Fig. 2).
GGU sample 301354 was collected by J.S. Peel on 17 August 1985 from the lower member of the Henson Gletscher Formation on a nunatak in southern Freuchen Land (82°10.5'N, 42°09'W; Fig. 2). GGU sample 271748 was collected by J.S. Peel on 16 July 1978 from about 3 m below the median sandstone member of the Henson Gletscher Formation, Løndal, Peary Land (82°17.5'N, 37°03'W; Fig. 2). The sample is located in the stratigraphic section illustrated by Peel et al. (Reference Peel, Streng, Geyer, Kouchinsky and Skovsted2016, fig. 2A). GGU sample 218584 was collected by J.R. Ineson on 28 July 1979 in southwest Peary Land, at the head of Henson Gletscher (82°10'N, 39°40'W; Geyer and Peel Reference Geyer and Peel2011, fig. 1D, E, locality 5; Blaker and Peel, Reference Blaker and Peel1997, fig. 8A, locality 4) from immediately below sandstones forming the upper 13 m of the formation (Fig. 2).
Repositories and institutional abbreviations
GGU prefix indicates a sample collected during field work by Grønlands Geologiske Undersøgelse (Geological Survey of Greenland), now a part of the Geological Survey of Denmark and Greenland, Copenhagen. Type and figured specimens from Greenland (PMU prefix) are deposited in the paleontological type collection of the Museum of Evolution, Uppsala University. Other repositories are noted in the text.
Systematic paleontology
Genus Tarimspira Yue and Gao, Reference Yue and Gao1992
1992 Tarimspira Reference Yue and GaoYue and Gao, p. 153.
1992 Fengzuella Reference He and YuHe and Yu, p. 5, non-Fengzuella Li and Han, Reference Li and Han1980.
1992 Otoformilites Qin and Li in Reference Ding, Zhang, Li and DongDing et al., p. 89.
2003 Fengzuella; Reference Steiner, Li, Qian, Zhu and ErdtmanSteiner et al., p. 858.
2015 Fengzuella; Reference Kouchinsky, Bengtson, Clausen and VendrascoKouchinsky et al., p. 479.
2015 Tarimspira; Yang et al. Reference Yang, Steiner and Kepp2015, p. 1561.
Type species
Tarimspira plana Yue and Gao, Reference Yue and Gao1992 from the Aksu-Wushi region, Xinjiang, China.
Diagnosis
Sclerites varying from bilaterally symmetrical, laterally compressed, planispirally coiled with up to three whorls, through shallowly curved and laterally compressed, to almost straight with circular cross section. Coiled morphotypes open coiled or with whorls in contact, often with a spiral keel or carina, or a shallow spiral depression, on periphero-lateral areas. Multilayered with growth lamellae overlapping lateral areas from the basal (abapical) surface. Lamellae compact externally but may be only loosely in contact internally, with cavities, and possibly a circumperipheral canal.
Occurrence
Cambrian Series 2 of China, Siberia, and Laurentia (Fig. 2.4).
Remarks
The diagnosis of Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003), itself an emendation, is abbreviated and modified to take account of the greater number of whorls in sclerites of Tarimspira artemi n. sp. Furthermore, the central cavity is not present in all sclerites assigned to Tarimspira herein.
Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) synonymized Tarimspira Yue and Gao, Reference Yue and Gao1992 and Otoformilites Qin and Li in Ding et al., Reference Ding, Zhang, Li and Dong1992 with Fengzuella He and Yu, Reference He and Yu1992 following analysis of the dates of their publication. The latter genus, however, is a junior homonym of Fengzuella Li and Han, Reference Li and Han1980 that prompted Yang et al. (Reference Yang, Steiner and Kepp2015) to propose Tarimspira as a replacement name for Fengzuella He and Yu, Reference He and Yu1992. Ironically, both Li and Han (Reference Li and Han1980) and He and Yu (Reference He and Yu1992) also employed zhejiangensis as the species-group epithet for their respective genera. On account of this species-group homonymy, Yang et al. (Reference Yang, Steiner and Kepp2015) gave taxonomic priority to Tarimspira plana Yue and Gao, Reference Yue and Gao1992, considering the entire combination Fengzuella zhejiangensis He and Yu, Reference He and Yu1992 to be invalid. However, application of International Code of Zoological Nomenclature (ICZN) article 57.8.1 states that “Homonymy between identical species-group names in combination (originally or subsequently) with homonymous generic names having the same spelling but established for different nominal genera is to be disregarded” (International Commission on Zoological Nomenclature, 1999, 2012). Fengzuella Li and Han, Reference Li and Han1980, an Ordovician brachiopod, and Fengzuella He and Yu, Reference He and Yu1992, a problematic Cambrian sclerite, are different nominal genera with the consequence that Tarimspira zhejiangensis (He and Yu, Reference He and Yu1992) is a valid name. It is employed in the present context for specimens described by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) from Zhejiang.
Contrary to the opinion of Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003), Tarimspira plana Yue and Gao, Reference Yue and Gao1992 is not considered to be a junior synonym of Tarimspira zhejiangensis (He and Yu, Reference He and Yu1992). Furthermore, the collective synonymization under Tarimspira plana proposed by Yang et al. (Reference Yang, Steiner and Kepp2015) is not accepted.
The vitreous, translucent, phosphatic composition of specimens from Greenland suggests that Tarimspira sclerites were composed of primary calcium phosphate. Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) and Kouchinsky et al. (Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015) suggested that Tarimspira phosphatized remains may have been originally unmineralized, as proposed for early Cambrian protoconodonts by Slater et al. (Reference Slater, Harvey and Butterfield2018).
Tarimspira plana Yue and Gao, Reference Yue and Gao1992
1992 Tarimspira plana Yue and Gao, Reference Yue and Gao1992, p. 153, pl. 5, figs. 7–9.
2015 Tarimspira plana; Reference Yang, Steiner and KeppYang et al., p. 1561, fig. 10O, P.
Holotype
Specimen 33055/BH7-3a, illustrated by Yue and Gao (Reference Yue and Gao1992, pl. 5, fig. 9a, b), Xiaoerbulak Formation (Cambrian Series 2, Stage 4), Aksu-Wushi Region, Tarim Basin, Xinjiang, northwest China (repository unknown).
Diagnosis
Tightly coiled, planispiral, and bilaterally symmetrical, with a pair of peripheral keels.
Description
The laterally compressed, planispiral, bilaterally symmetrical sclerite is tightly but uniformly coiled through about one and a quarter whorls. Shallowly convex lateral surfaces become concave prior to their transition into a pair of robust spiral keels, located at the periphery, one on each side of the plane of symmetry. The peripheral keels become reduced in relief at the latest growth stage. They delimit a median channel that is slightly V-shaped in transverse profile, with shallowly convex sides, in the early growth stages (Fig. 1.3) but becomes flattened at the latest growth stage (Fig. 1.4). Adaxially, each lateral surface curves abruptly into a deep narrow umbilicus formed as the growing sclerite overlaps earlier growth stages. At the latest growth stage, the sutural area between the enveloping whorl and the earlier growth stage consists of a deep invagination, with the latest growth lamellae not in direct contact with the earlier whorl (Fig. 1.1).
Externally, the sclerite is compact and appears to be solid, formed by a series of lamellae that slope obliquely abapically in from the lateral areas toward the axial plane in the direction of growth. Thus, the latest growth lamella forms a continuous surface that curves around the growing margin of the sclerite from one lateral area to the other (Fig. 1.5, arrow). The lamellae produce regular transverse lines of growth that are concave toward the direction of growth of the sclerite.
Materials
PMU 31847 from GGU sample 255522, Aftenstjernesø Formation, Cambrian Series 2, Stage 4, Løndal, Peary Land, North Greenland.
Remarks
In following discussion, the morphology of this sclerite is referred to as morphotype A (Fig. 3.8).
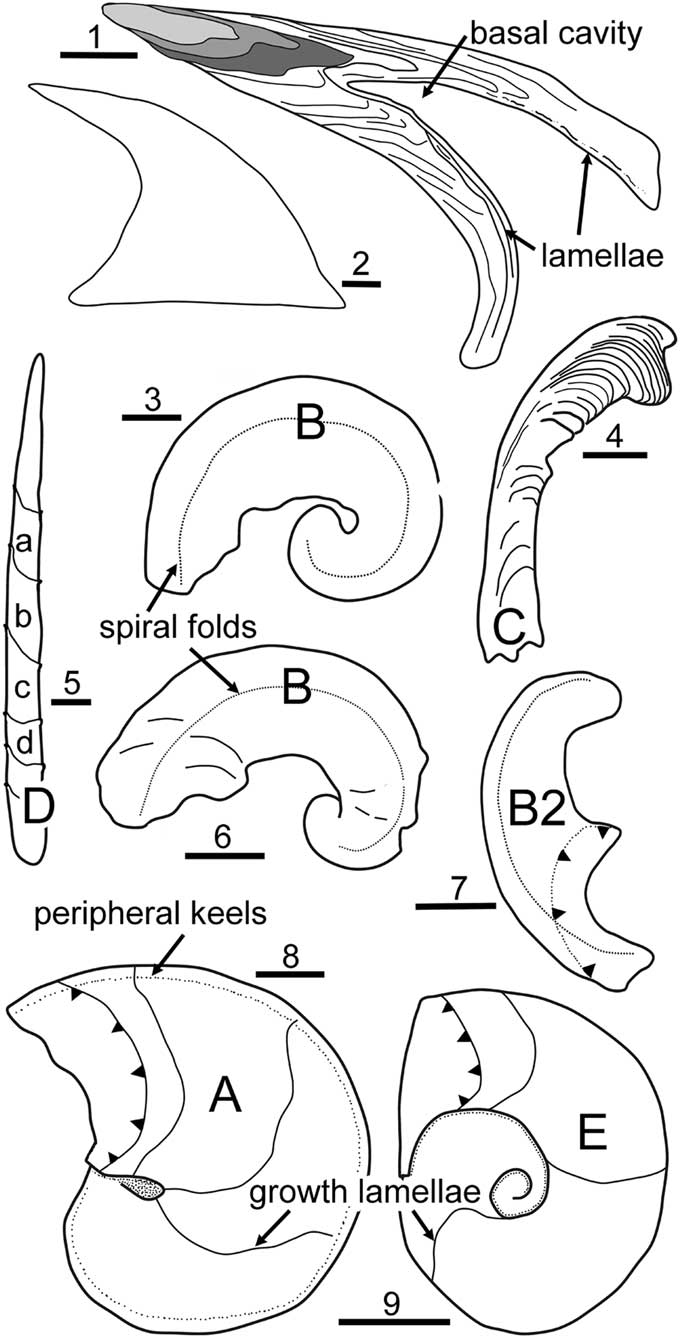
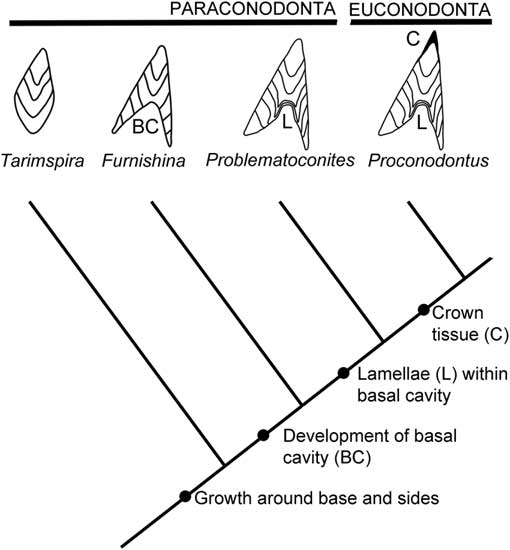
Figure 3 (1, 2) Problematoconites, cross section and outline of complete paraconodont element from the Windfall Formation, Ordovician (Tremadocian), Nevada, USA, showing early growth stages (shaded) with basal growth lamellae overlapping lateral areas and lamellae that subsequently traverse the entire basal cavity (sketched after Murdock et al. Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013). (3–6) Tarimspira zhejiangensis (He and Yu, Reference He and Yu1992), early Cambrian, Zhejiang, China, sketched from photographs by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003); (3) holotype of Fengzuella zhejiangensis He and Yu, Reference He and Yu1992, specimen 89-11-2-2452 in the Nanjing Institute of Geology and Palaeontology, China, morphotype B, Hetang Formation; (4) morphotype C, Dui2-1-12, chert unit beneath Hetang Formation; (5) morphotype D, showing overlapping lamellae (a–d, oldest to youngest) sketched from Dui2-2-7a and Dui2-2-26, chert unit beneath Hetang Formation; (6) morphotype B, 133-2-15, chert unit beneath Hetang Formation. Specimens other than the holotype are deposited in the Technical University (Berlin). (7, 9) Tarimspira artemi n. sp., Henson Gletscher Formation, North Greenland, with adapical margin of latest growth lamella marked with arrow heads; (7) morphotype B2; (9) morphotype E. (8) Tarimspira plana Yue and Gao, Reference Yue and Gao1992, Aftenstjernesø Formation, North Greenland, morphotype E, with adapical margin of latest growth lamella marked with arrow heads. (1, 3–9) Scale bars = 50 µm; (2) scale bar = 100 µm.
The embracive synonymy of previously described taxa with Tarimspira plana Yue and Gao, Reference Yue and Gao1992 proposed by Yang et al. (Reference Yang, Steiner and Kepp2015) is not accepted. Yue and Gao (Reference Yue and Gao1992) described Tarimspira plana from the lowermost beds of the Xiaoerbulak Formation. Two of the specimens illustrated by Yue and Gao (Reference Yue and Gao1992, pl. 5, figs. 8, 9) show the bilaterally symmetrical form of the Greenland specimen. In a third specimen, the keels are displaced to one side and bilateral symmetry is lost, suggesting that this may be a second sclerite type within the scleritome.
While the outer surface of the only known specimen from Greenland is compact, the broken peripheral tip at the latest growth stage reveals an inner cavity within several widely spaced lamellae (Fig. 1.5). It is not known whether this cavity is partly original or a function of preservation. Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) described similar loose contact between internal lamellae in Tamispira zhenjaingensis and inferred the presence of a peripheral internal canal, discussed in the folllowing.
The holotype illustrated by Yue and Gao (Reference Yue and Gao1992) from the Xiaoerbulak Formation (Cambrian Series 2, Stage 4) of the Aksu-Wushi region of Xinjiang, northwest China, is twice the length of the single sclerite known from the Aftenstjernesø Formation. Two specimens illustrated by Yang et al. (Reference Yang, Steiner and Kepp2015) from the Shuijingtuo Formation (Cambrian Series 2, Stage 3) of South China are even larger. In one of these (Yang et al., Reference Yang, Steiner and Kepp2015, fig. 10P), the whorl uncoils in its latest fraction. Coiling becomes tighter in the other specimen, occluding the umbilici (Yang et al., Reference Yang, Steiner and Kepp2015, fig. 10O), but it seems likely that this may be a variable character.
Tarimspira zhejiangensis (He and Yu, Reference He and Yu1992)
1992 Fengzuella zhejiangensis Reference He and YuHe and Yu, p. 5, pl. 2, fig. 17 non–Fengzuella zhejiangensis Li and Han, Reference Li and Han1980.
2003 Fengzuella zhejiangensis; Reference Steiner, Li, Qian, Zhu and ErdtmanSteiner et al., p. 38, fig. 3a, b, p, q.
Holotype
Specimen number 89-11-2-2452 in the Nanjing Institute of Geology and Palaeontology illustrated by He and Yu (Reference He and Yu1992) and by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003, fig. 3a, b) from the Hetang Formation, Jiangshan County, Zhejiang Province, China.
Remarks
Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) described three types of sclerite (here referred to as morphotypes B–D; Fig. 3) within the scleritome of Tarimspira zhejiangensis (as Fengzuella zhejiangensis). The eponymous sclerite (morphotype B; Fig. 3.3, 3.6) is rare in the assemblages from the Hetang Formation. They are dominated numerically by sclerites of morphotype C (Fig. 3.4), which can be considered to characterize the scleritome.
Tarimspira artemi new species

Figure 4 Tarimspira artemi n. sp., morphotype E, Henson Gletscher Formation, Cambrian Series 2, Stage 4; GGU sample 301354, southern Freuchen Land, North Greenland. (1) PMU 31849; (2, 8) PMU 31848, holotype, with arrow in (2) indicating growth lamellae extending from the base onto the lateral areas; (3) PMU 31850, broken specimen with circumperipheral cavity on penultimate whorl (arrow); (4, 5) PMU 31851; (6, 7) PMU 31852. (1–6, 8) Scale bars = 50 µm; (7) scale bar = 25µm.
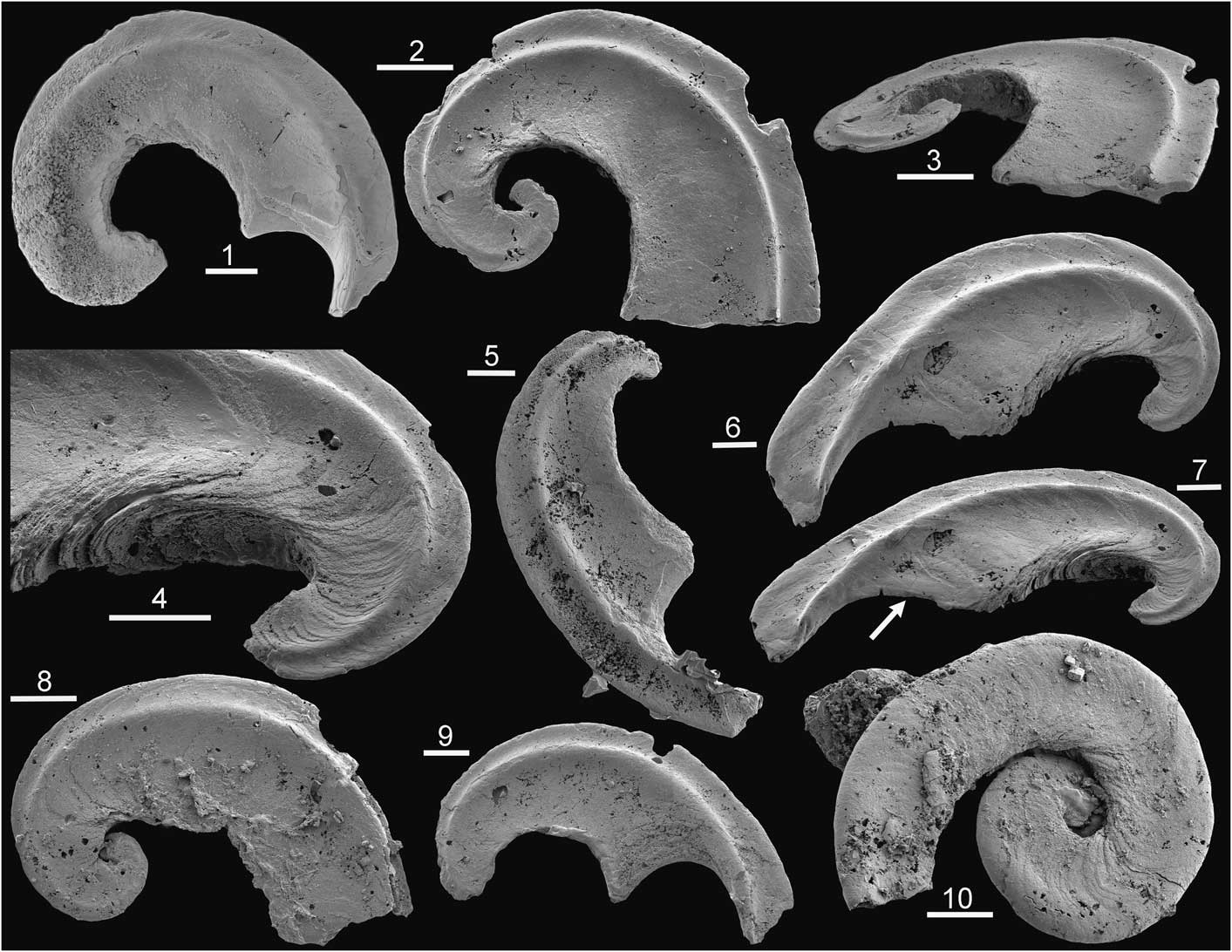
Figure 5 Tarimspira artemi n. sp., Henson Gletscher Formation, Cambrian Series 2, Stage 4; GGU sample 301354, southern Freuchen Land, North Greenland, unless stated. Morphotype B2 unless stated. (1) PMU 31853; (2, 3) PMU 31854; (4, 6, 7) PMU 31855, with arrow in (7) indicating the last deposited growth lamella extending from the base onto the lateral areas; (5) PMU 31856; (8) PMU 31858 from GGU sample 271748 Løndal, Peary Land, morphotype B; (9) PMU 31857; (10) PMU 31859 from GGU sample 218584, Henson Gletscher Formation, Henson Gletscher, southwest Peary Land, morphotype E. Scale bars = 50 µm.
2015 Fengzuella zhejiangensis; Reference Kouchinsky, Bengtson, Clausen and VendrascoKouchinsky et al., p. 479, fig. 54.
Holotype
PMU 31848 from GGU sample 301354, Henson Gletscher Formation, Cambrian Series 2, Stage 4, southern Freuchen Land, North Greenland.
Diagnosis
Laterally compressed, planispiral, bilaterally symmetrical sclerite that is tightly but uniformly coiled through about one and a half to three whorls; lateral areas shallowly concave, without spiral keels or carinae (based on sclerite morphotype E).
Description
The diagnostic sclerite (morphotype E) is laterally compressed, planispiral, bilaterally symmetrical and uniformly coiled through about one and a half to three whorls. The lateral surfaces are shallowly concave between the rounded periphery and the umbilical shoulders; spiral keels or carinae are absent. Adaxially, each lateral surface curves abruptly into a wide umbilicus formed as the growing sclerite overlaps earlier growth stages. The sutural area between the enveloping whorl and the earlier growth stage is deeply invaginated.
Externally, the sclerite is compact and appears to be solid. Growth lamellae slope obliquely abapically in from the lateral areas toward the axial plane in the direction of growth. Each growth lamella forms a continuous surface that curves from one lateral area to the other around the growing margin (base) of the sclerite (arrows in Figs. 4.2, 5.7). The growth lamellae produce regular transverse lines of growth that are concave toward the direction of growth (base) of the sclerite. Adjacent to the periphery, growth lamellae curve forward to form an elevation on the basal surface and appear to delimit a circumperipheral tubular canal (arrow in Fig. 4.3, 4.6, 4.7).
A single specimen similar to the holotype of Tarimspira zhejiangensis was found in the Henson Gletscher Formation of Løndal (Fig. 5.8), but it is tentatively referred to Tarimspira artemi in the absence of any other associated sclerites.
The associated sclerite (morphotype B2; Fig. 5.1–5.7, 5.9) is laterally compressed, bilaterally symmetrical, and typically open coiled through about a half to a full whorl; it is characterized by a prominent spiral carina with concave sides on each lateral surface close to the acute periphery (Fig. 5.2).
Etymology
For Artem Kouchinsky in recognition of his contribution to the study of Cambrian faunas.
Materials
PMU 31849–PMU 31857 from GGU sample 301354, southern Freuchen Land; PMU 31858 from GGU sample 271748, Løndal; PMU 31859 from GGU sample 218584, Løndal. Henson Gletscher Formation, Cambrian Series 2, Stage 4, North Greenland.
Remarks
The diagnostic morphotype (morphotype E) is distinguished from Tarimspira plana (morphotype A) by its wider umbilici and lack of spiral keels around the periphery. The associated sclerite (morphotype B2) resembles sclerite morphotype B of Tarimspira zhejiangensis in terms of the prominent spiral carinae on the lateral surfaces, but most specimens are more openly coiled through less than a whorl (Fig. 5.5, 5.6). However, a single specimen from GGU sample 271748 in Løndal (Fig. 5.8), tentatively placed here, is more strongly coiled and therefore morphologically close to the holotype of Tarimspira zhejiangensis (Fig. 3.3).
The nature of the possible circumperipheral canal in Tarimspira artemi is obscure, not least since it is developed at the apex of the subperipheral elevation (Fig. 4.6). It may be an artifact produced by fracturing, or imperfect stacking of growth lamellae that fail to maintain contact just at their apices in the cone-in-cone structure (Fig. 4.7). Gaps between lamellae may also result in part from diagenesis or etching during preparation, as visible in the B2 morphotype (Fig. 5.4). Well-preserved specimens of this latter morphotype show no evidence of a canal (Fig. 5.6, 5.7). Spine-like sclerites (Fig. 3.5, morphotype D) assigned to Tarimspira zhejiangiensis by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003, fig. 3m–o, s) seem to have a hollow core, but the separation of the spiral lamella suggests a diagenetic origin. However, Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003, fig. 3f) described a circumperipheral central cavity extending from the base to the apex in morphotype C (Fig. 3.4), although the relationship of this to the basal part of the growth lamellae is unclear.
Sclerite growth
Sclerites of problematic organisms are diverse and abundant in assemblages of shelly fossils from the lower Cambrian (Bengtson, Reference Bengtson2005; Rozanov et al., Reference Rozanov2010; Kouchinsky et al., Reference Kouchinsky, Bengtson, Runnegar, Skovsted, Steiner and Vendrasco2011, Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015), but Tarimspira sclerites differ from most described examples in their mode of secretion; they are constructed of abapically sloping lamellae (Figs. 1.5, 1.6, 3.7–3.9). Thus, the latest (basal) growth lamella was deposited externally; it overlies the previously formed lamella as it passes continuously from one lateral surface to the other around the abapical (basal) extremity (Fig. 1.5, arrow). As noted by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) and Kouchinsky et al. (Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015), this growth pattern indicates that the developing sclerites were rooted in an invagination in underlying soft tissues. Contemporaneous Cambrian sclerites such as lapworthellids (Devaere and Skovsted, Reference Devaere and Skovsted2017) and protoconodonts (Szianiawski, Reference Szaniawski1982, Reference Szaniawski1983, Reference Szaniawski1987, Reference Szaniawski2002; Bengtson, Reference Bengtson1983; McIlroy and Szaniawski, Reference McIlroy and Szaniawski2000; Vannier et al., Reference Vannier, Steiner, Renvoisé, Hu and Casanona2007) grew by the addition of lamellae on the inside of a hollow basal cavity, with deposition essentially upon papillae within the basal cavity. Both methods of growth increase the anchoring surface area of attachment relative to a simple, smaller, planar contact, which may enhance their defensive role or, in spinose grasping sclerites such as those of protoconodonts, even their maneuverability.
Comparable incremental growth around the lateral areas and across the base of sclerites occurs in paraconodonts such as Furnishina Müller, Reference Müller1959, Prooneotodus Müller and Nogami, Reference Müller and Nogami1971, and Problematoconites Müller, Reference Müller1959 (Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013; Fig. 3.1, 3.2), although the acute spinose form and deep basal cavity of these paraconodonts find no morphological equivalence in Tarimspira. Instead of a basal cavity, the abapical termination of most sclerites of Tarimspira is rounded or wedge shaped (arrows in Fig. 1.5, 1.6; Fig. 5.1–5.7). The abapically concave shape of the growth lamellae as they cross the lateral areas of the sclerite in Tarimspira plana indicates that mineralizing tissue embraced the sclerite mainly on its lateral areas such that the earlier formed coil and umbilical areas in Tarimspira plana, together with most of the supra-apical surface, were probably exposed, at least periodically. While sclerite formation was within an invagination, the shape of the growth lamellae indicates that this pocket was likely formed as a cleft traversing either a papilla or a transverse ridge of secreting tissues.
The growing margin (abapical termination of the sclerite) is clearly visible in Tarimspira artemi n. sp. (arrow in Fig. 4.2) and in sclerites with pronounced lateral spiral carinae (Fig. 5.1, 5.6, 5.9). Lateral ridges in the latter form a prominent transverse element that both reinforces the narrow sclerite and stabilizes its attachment in the soft tissues perpendicular to the otherwise narrow attachment surface along the plane of symmetry.
The prominent spiral lateral carinae of some Tarimspira sclerites (Fig. 5.1–5.9) are reminiscent of the lateral costae of distacodiform euconodont elements (Robison, Reference Robison1981), but such structures are not typical of paraconodonts of the Furnishina–Prooneotodus–Problematoconites morphological group. In Coelocerodontus Ethington, Reference Ethington1959, however, the tall, laterally compressed, thin-walled conical element may develop a prominent carina on either or both lateral surfaces and a pair of spiral keels on the supra-apical surface (Andres, Reference Andres1988; Dong and Zhang, Reference Dong and Zhang2017). Specimens of Coelocerodontus from the early Ordovician of Sweden illustrated by Andres (Reference Andres1988, fig. 18) show a similar degree of curvature, rate of expansion, and ornamentation of spiral carinae to some sclerites of Tarimspira (Fig. 5.5, 5.6). Andres (Reference Andres1988) considered Coelocerodontus to be a paraconodont. Szaniawski (Reference Szaniawski2015) suggested a separate group on the basis of its histology, but Dong and Zhang (Reference Dong and Zhang2017) assigned it to the euconodonts. While the development of carinae perpendicular to the plane of symmetry in Tarimspira, Coelocerodontus, and distacodiform euconodont elements may suggest an evolutionary relationship, it is more likely that it reflects the common functions of strengthening the sclerite and increasing its anchorage in secreting soft tissues.
A basal cavity is not present in Tarimspira where the basal surface is usually protruding. There is evidence of the presence of a narrow spiral internal canal extending from the basal surface toward the apex in some sclerites (Fig. 4.6), as also discussed by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003), but the nature of the canal and, if present, its function are uncertain. A relationship to the basal cavity of members of the Furnishina–Prooneotodus–Problematoconites morphological group might be suggested, although the initial growth stages of their elements do not show a comparable structure (Andres, Reference Andres1988; Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013).
Scleritome
Reconstruction of isolated sclerites into their original scleritomes is a vital step in the determination of the affinity of Cambrian problematic organisms. The special preservation offered by Lagerstätten has proved invaluable with regard to groups such as the chancelloriids (Bengtson and Collins, Reference Bengtson and Collins2015) and halkieriids (Conway Morris and Peel, Reference Conway Morris and Peel1995). However, the reconstruction of many other organisms remains speculative and often a mental challenge, as demonstrated, for example, by the multiplated Trachyplax arctica Larsson, Peel, and Högstrom, Reference Larsson, Peel and Högström2009 that occurs in North Greenland in co-eval strata to those yielding the unrelated Tarimspira plana (Larsson et al., Reference Larsson, Peel and Högström2009).
Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) noted, but did not illustrate, clusters of similar sclerites of Tarimspira (as Fengzuella) zhejiangiensis in samples from the early Cambrian of South China (Fig. 3.4–3.6) but no recognizable scleritomes. However, they recognized three co-occurring sclerite morphotypes, here referred to as morphotypes B–D (Fig. 3; the tightly coiled sclerites of Tarimspira plana are designated as morphotype A). Coiled sclerites of morphotype B (Fig. 3.6) are rare. They are usually curved through slightly more than one revolution and more open coiled than the holotype of Fengzuella zhejiangensis He and Yu, Reference He and Yu1992, refigured by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003, fig. 3a, b; Fig. 3.3, 3.6), but otherwise similar. Morphotype B sclerites carry a prominent spiral fold on each lateral area.
More than 85% of the specimens available to Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) are laterally compressed curved sclerites (length 410–1,340 µm) with subparallel sides, a uniformly curved supra-apical surface, and a narrow base (morphotype C). These curved sclerites are coiled through less than half a whorl in lateral perspective (Fig. 3.4), with a median keel along the supra-apical surface, but lack spiral folds or ridges on the lateral areas (Steiner et al., Reference Steiner, Li, Qian, Zhu and Erdtman2003, fig. 3c). Narrow, straight or only slightly curved sclerites (morphotype D) are pointed and attain greater length than the curved sclerites. While they show the characteristic overlapping lamellae (Fig. 3.5), a cross section illustrated by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003, fig. 3s) indicates that this is a continuous spiral lamella rather than a series of discrete, stacked, cone-shaped lamellae. This difference in the mechanism of growth from morphotypes A–C makes it uncertain whether this sclerite belongs to the Tarimspira zhejiangiensis scleritome, although the two forms may represent end members in a morphological series. The sclerite in morphotypes A–C is planispirally coiled, without any translation along the axis (Fig. 3). This coiling is tight in morphotype A but widely open coiled in morphotype C. In the spine-like morphotype D, there is a high rate of translation of the growing lamella along the axis of coiling with the result that the direction of growth of the sclerite approximates to the orientation of the coiling axis. Growth increase in morphotypes A and B is perpendicular to the axis of coiling.
The increase in translation in morphotype D thus provides a mechanism for the development of a spinose sclerite with a possible grasping function. While its function may parallel the grasping function of sclerites of protoconodonts (Szaniawski, Reference Szaniawski1983) and panderodid eucondodonts (Sansom et al., Reference Sansom, Armstrong and Smith1995), the growth of morphotype D is constrained by the underlying protruding base characteristic of Tarimspira.
Greenland material assigned to Tarimspira artemi n. sp. includes two sclerite morphotypes. Curved to open-coiled sclerites (Fig. 3.7, morphotype B2; Fig. 5.1–5.9) are similar to the morphotype B sclerites of Tarimspira zhejiangensis (Figs. 3.3, 4.6) and carry a prominent spiral ridge on either side of the acute supra-apical keel. They occur together with tightly coiled sclerites in which coiling varies between two and three whorls, but which lack spiral ridges on the lateral areas (Fig. 3.9, morphotype E; Fig. 4). Sclerites of this type are not described in Tarimspira zhejiangensis, but both sclerite morphotypes B2 and E are recognized in material from the Emyaksin Formation of northern Siberia described by Kouchinsky et al. (Reference Kouchinsky, Bengtson, Clausen and Vendrasco2015, fig. 54), which is here assigned to Tarimspira artemi n. sp. The single specimen of Tarimspira plana known from Greenland (Fig. 3.8, morphotype A; Fig. 1) is the same morphotype as the tightly coiled holotype (Yue and Gao, Reference Yue and Gao1992) and specimens figured by Yang et al. (Reference Yang, Steiner and Kepp2015), but the former authors also illustrated an open-coiled morphotype with a lateral ridge similar to that noted here in morphotype B of Tarimspira zhejiangensis (Fig. 3.3, 3.6) and in Tarimspira artemi (morphotype B2; Fig. 5.1–5.9). Thus, at least partial scleritomes with distinctive sclerites can be recognized for each of the three described species of Tarimspira (T. plana, T. zhejiangensis, and T. artemi), although it is likely that these scleritomes contain other as yet unknown sclerite morphotypes.
The arrangement of individual sclerites and their precise function within the scleritomes are not known. Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) speculated that sclerites were closely packed in Tarimspira zhejiangensis with morphotypes B and C located laterally to morphotype D. Interpretation of the sclerites as dorsal armor might promote comparison with well-known but unrelated forms such as the early–middle Cambrian Wiwaxia Walcott, Reference Walcott1911 (Slater et al., Reference Slater, Harvey, Guilbaud and Butterfield2017, fig. 14) or Halkieria Poulsen, Reference Poulsen1967 (Conway Morris and Peel, Reference Conway Morris and Peel1995), although the morphology, method of formation, and composition of individual sclerites in the latter are fundamentally different (Smith, Reference Smith2014; Zhang et al., Reference Zhang, Smith and Shu2015). Furthermore, there is little direct evidence concerning the orientation of sclerites in Tarimspira.
The present comparison with paracondonts in terms of sclerite growth promotes interpretation of the sclerites of Tarimspira as elements within an oropharyngeal feeding array (Donoghue et al., Reference Donoghue, Forey and Aldridge2000), but there is little morphological similarity with known arrays. Andres (Reference Andres1988, fig. 17) presented a partial reconstruction of Coelocerodontus that might be applied to sclerites of morphotype B2, although these are more curved and less pointed than elements of Coelocerodontus and lack any obvious grasping function. However, this function may have been provided by the spine-like morphotype D. The position, orientation, and function of the tightly coiled sclerites of morphotypes A and E in such a reconstruction are unknown, but they may have had a crushing function deep in the pharynx in contrast to the grasping function of elements of the Furnishina–Prooneotodus–Problematoconites type (Murdock et al., Reference Murdock, Rayfield and Donoghue2014). Whereas the basal (proximal) surface of sclerites probably would be located anteriorly in an elongate dorsal scleritome, with the curvature toward the posterior, no preferred single orientation can be assumed in oropharanygeal conodont arrays (Sansom et al., Reference Sansom, Armstrong and Smith1995; Szaniawski, Reference Szaniawski2002; Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013).
Systematic position
The style of basal accretion with lateral overlap promotes comparison of Tarimspira with paraconodonts, but there is little similarity in terms of overall morphology between Tarimspira and fully developed paraconodont elements (Müller, Reference Müller1959; Müller and Nogami, Reference Müller and Nogami1971; Andres, Reference Andres1988; Müller and Hinz, Reference Müller and Hinz1991; Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013), suggesting that the arrangement and function of the respective hard parts were disimilar. The morphological disparity may imply that the unusual method of accretion represents convergence in the method of growth, although a different but equally great range in morphology is seen when comparing other groups widely accepted as paraconodonts, namely the arcane westergaardodinids (Müller, Reference Müller1959; Müller and Hinz, Reference Müller and Hinz1991) and the slender, curved, simple cones with a deep basal cavity extending almost to the tip seen in Furnishina, Prooneotodus, and Problematoconites (Fig. 3.1, 3.2).
Sclerites of Tarimspira lack a basal cavity, but the basal cavity in paraconodonts such as Furnishina, Prooneotodus, and Problematoconites is not developed until after elements attain a length of 100–300 µm (Andres, Reference Andres1988, fig. 19; Murdock et al., Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013, fig. 2; Fig. 3.1). Prior to this stage, the characteristic basal overlap of the lateral areas by successive accreted layers closely resembles that seen in Tarimspira (Figs. 1.5, 5.4). Thus, Tarimspira sclerites could be interpreted as early ontogenetic stages of paraconodonts formed prior to the development of a basal cavity, although this interpretation is opposed by the large size of Tarimspira elements (1.5 mm) reported by Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtman2003) and the lack of direct evidence of later ontogenetic stages with a semblance of a basal cavity in any of the samples.
It is proposed that Tarimspira sclerites may reflect a stage in paraconodont evolution prior to the development of a basal cavity, suggesting a simple expansion of the model of paraconodont–euconodont evolution proposed by Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013; Fig. 6). As concerns Tarimspira, however, the model is simplistic in that it is mainly based on the form of morphotypes A, B, and B2 (Fig. 3). Morphotype C is readily interpreted as a more open-coiled version of morphotype B2. Morphotype D of Tarimspira zhejiangensis is bizarre and may not belong to the scleritome, although greatly increased translation along the coiling axis is a feasible explanation of its growth form.

Figure 6 Proposed phylogenetic relationship between Tarimspira, paraconodonts, and euconodonts, based on Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013, fig. 4).
The interpretation of Tarimspira as a paraconodont is consistent with the geological record and pushes the known record of paraconodont vertebrates back into the early Cambrian. The oldest previously recognized paraconodonts, represented by Furnishina in the model of Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013), are reported from the Ptychagnostus gibbus Biozone (uppermost Stage 5) of Cambrian Series 3 (Dong and Bergström, Reference Dong and Bergström2001; Dong et al., Reference Dong, Repetski and Bergström2001; Kouchinsky et al., Reference Kouchinsky, Bengtson, Runnegar, Skovsted, Steiner and Vendrasco2011; Dong and Zhang, Reference Dong and Zhang2017) whereas Tarimspira occurs in Cambrian Series 2 (Stage 4).
Paraconodonts thus encompass at least three distinct morphological groups: the Furnishina–Prooneotodus–Problematoconites slender cones with deep basal cavities, the westergaardodinids, and Tarimspira. The inhomogeneity suggests that the relationships of these groups of early vertebrates are poorly understood, adding substance to the demonstration of paraphyly by Murdock et al. (Reference Murdock, Dong, Repetski, Marone, Stampanoni and Donoghue2013).
Acknowledgments
M. Steiner (Berlin) helped with literature from China. A. Kouchinsky (Stockholm) prepared some of the original scanning electron micrographs. B.J. Slater (Uppsala) is thanked for discussion and comments on the original manuscript. T.H.P. Harvey (Leicester) and D.J.E. Murdock (Oxford) are thanked for thoughtful reviews of the submitted manuscript.