Introduction
The overall life expectancy is increasing worldwide. As older people are typically more vulnerable to frailty and chronic conditions, such as dementia, a rise in the incidence and prevalence of Alzheimer's disease (AD) is expected (Graham et al., Reference Graham1997).
According to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) criteria, the diagnosis of probable AD includes insidious onset and progressive impairment of memory and other cognitive functions, such as orientation, language, praxis, attention, visual perception, and executive function (McKhann et al., Reference Mckhann, Drachman, Folstein, Katzman, Price and Stadlan1984). Therefore, the evaluation of cognitive function is a crucial part of the dementia diagnosis process. Most studies on oral communication in AD focus on aphasia (Vuorinen et al., Reference Vuorinen, Laine and Rinne2000; de Lira et al., Reference De Lira, Ortiz, Campanha, Bertolucci and Minett2011); however, speech and orofacial apraxias are also present in these patients (Croot et al., Reference Croot, Hodges, Xuereb and Patterson2000; Gerstner et al., Reference Gerstner, Lazar, Keller, Honig, Lazar and Marshall2007). Aphasia is defined as the acquired impairment of language processes underlying receptive and expressive modalities (Akbarzadeh and Moshtagh-Khorasani, Reference Akbarzadeh and Moshtagh-Khorasani2007), whereas apraxia is an impairment in the ability to perform purposeful movement (Pedretti et al., Reference Pedretti, Zoltan, Wheatley and Pedretti1996).
More specifically, speech apraxia is a disturbance that interferes with the capacity to program the positioning and sequencing of muscle movements for producing phonemes (Darley, Reference Darley1969), and orofacial apraxia is a specific type of ideomotor apraxia in which there is an impairment in the non-verbal movements of the face, lips, tongue, and pharynx following a verbal command or imitation (Broussolle et al., Reference Broussolle1996).
Many aphasic, apraxic, and dysarthric disorders occur as a result of extensive lesions that impair multiple cognitive systems resulting in “aphasia with apraxia of speech” or “apraxia of speech with dysarthria” (Croot, Reference Croot2002). Broussolle et al. (Reference Broussolle1996) found that orofacial and speech apraxias co-occur because of the anatomical proximity of structures involved in their appearance. The authors reported cortical atrophy mostly restricted to the left frontal cortex; the anterior operculum and premotor and sensorimotor cortices were the most affected areas in a neuroimaging of eight patients who presented with a clinically recognizable syndrome of progressive speech impairment without dementia (Broussolle et al., Reference Broussolle1996). Apraxia of speech in stroke cases can occur due to the left superior precentral gyrus of the insula (Ogar et al., Reference Ogar, Willock, Baldo, Wilkins, Ludy and Dronkers2006). In patients with AD who demonstrate that phonological and articulatory impairments, neuropathological changes were located in regions of brain frontal, temporal, parietal, and left perisylvian areas (Croot et al., Reference Croot, Hodges, Xuereb and Patterson2000). There is significant global atrophy in AD (Baron et al., Reference Baron2001); therefore, multiple communication disorders could be expected. As for the brain areas affected in this disease, Baron et al. (Reference Baron2001) reported that in mild AD, in approximate decreasing order of statistical significance, gray matter loss affects the anterior amygdala and hippocampus/entorhinal cortex areas, the posterior cingulate cortex and adjacent precuneus, perisylvian areas, the temporoparietal association neocortex, the posterior hippocampus, the anterior hypothalamus and thalamus, the prefrontal cortex, and the caudate nucleus and putamen, with only slight atrophy in the frontal lobe. Gerstner et al. (Reference Gerstner, Lazar, Keller, Honig, Lazar and Marshall2007) presented a case of pathologically proven AD with mood disturbances and progressive speech loss consistent with speech apraxia. The postmortem examination disclosed AD pathology in the hippocampal formation, basal nucleus of Meynert and neocortex with neuritic plaques in the frontal, occipital, and inferior temporal gyri. Baron et al. (Reference Baron2001) and Gerstner et al. (Reference Gerstner, Lazar, Keller, Honig, Lazar and Marshall2007) showed that AD affects areas which are also involved in the manifestation of speech apraxia, as described by Broussolle et al. (Reference Broussolle1996) and Croot et al. (Reference Croot, Hodges, Xuereb and Patterson2000).
In AD, syntactic and semantic linguistic changes are often studied because they are more evident and cause greater functional impairment in the patient's verbal communication (de Lira et al., Reference De Lira, Ortiz, Campanha, Bertolucci and Minett2011) compared to apraxic manifestations. This approach seems to contribute to the neglect of studying speech and orofacial apraxias in AD. Although described in patients with definitive diagnosis of AD, the reports related to speech and orofacial apraxias in AD are limited to descriptions of cases with focal presentations of the disease (Kawamura and Mochizuki, Reference Kawamura and Mochizuki1999; Gerstner et al., Reference Gerstner, Lazar, Keller, Honig, Lazar and Marshall2007). In other neurodegenerative diseases, such as frontotemporal lobar degeneration, speech apraxia is one of the most striking features and results in serious functional impairment (Ogar et al., Reference Ogar, Dronkers, Brambati, Miller and Gorno-Tempini2007). However, speech and orofacial apraxias should also be considered in AD, as they limit patients’ oral communication and may interfere with communication rehabilitation. The current study will be the first to move from case series descriptions to the investigation of praxis in a larger sample of patients with AD, which is especially important when planning strategies for communication therapy.
The aim of this study was to investigate the presence of speech and orofacial apraxias in AD patients and test the hypothesis that apraxia severity is correlated to disease severity.
Methods
This was a cross-sectional study of 90 elderly patients with AD: 30 patients had mild AD, 30 moderate, and 30 severe. Dementia severity was classified according to the Clinical Dementia Rating (CDR) scale (Morris, Reference Morris1993; Montano and Ramos, Reference Montano and Ramos2005). All patients were referred from the outpatient unit of the behavioral neurology sector of the Department of Neurology and Neurosurgery of the Federal University of São Paulo.
Ethical approval was granted for this study (0390/08). Eligible patients or their legal guardians received information about the study, and those who gave informed consent were included.
The inclusion criteria were as follows: age ≥60 years, probable diagnosis of AD according to the clinical NINCDS-ADRDA criteria (McKhann et al., Reference Mckhann, Drachman, Folstein, Katzman, Price and Stadlan1984), and the presence of an informant. Patients with mild or moderate AD received therapeutic doses of cholinesterase inhibitors (donepezil ≥5 mg, rivastigmine ≥9 mg, or galantamine ≥8 mg) (Birks, Reference Birks2006). Dependency in instrument activities of daily living (IADL) was confirmed by the Lawton index (Lawton and Brody, Reference Lawton and Brody1969).
The exclusion criteria were as follows: independence in ADL; a history of alcoholism or use of illicit drugs; the use of psychoactive drugs at least once in the last month, with the exception of atypical neuroleptic drugs; previous severe neurologic or psychiatric disease (e.g. epilepsy, carcinoma, schizophrenia); visual or hearing impairments that could compromise assessment task performance; the absence of speech or inability to complete the evaluation, as the patient had to be able to respond to the stimuli presented to allow speech analysis.
All patients were evaluated by a multidisciplinary team composed of neurologists, responsible for medical history and physical examination; psychologists, responsible for the neuropsychological assessment; and a speech therapist who was responsible for the implementation of the speech therapy protocol related to this research.
Cognitive assessment
• The CDR adapted by Morris (Reference Morris1993) was used to classify dementia severity according to the Brazilian version validated by Montano and Ramos (Reference Montano and Ramos2005).
• The Mini-Mental State Examination (MMSE), Brazilian version validated by Brucki et al. (Reference Brucki, Nitrini, Caramelli, Bertolucci and Okamoto2003), was employed to evaluate global cognitive function.
• The oral agility subtest of the Boston Diagnostic Aphasia Examination (BDAE) was used to evaluate speech and orofacial praxis (Goodglass and Kaplan, Reference Goodglass and Kaplan1983). This test includes six tasks of orofacial agility and seven involving speech agility. The patients’ BDAE scores were compared with the Brazilian norms of 50 healthy volunteer sample aged 51 years or more (with 7.8±4.8 of the mean educational level): 8.7±2.2 points for orofacial agility and 12.1±1.9 for speech agility (Radanovic et al., Reference Radanovic, Mansur and Scaff2004). The minimum score is zero and the maximum scores for orofacial and speech agility are 12 and 14, respectively. Patients with scores below 7 and 11 were considered as having orofacial and speech apraxias, respectively.
• To better characterize the participants’ praxis, speech was also evaluated with the Martins and Ortiz (M&O) protocol for speech and orofacial praxis assessment (Martins and Ortiz, Reference Martins and Ortiz2004). The orofacial tasks correspond to a range of 0–200 points. Patients with scores below 160 were considered as having orofacial apraxia. The test comprises of the performance of 20 isolated and sequential movements in response to a verbal command (e.g. lip protrusion, lateralization of the tongue, protrusion and retraction of the lips, and lateralization and elevation of the tongue). When necessary, the movement was demonstrated by the examiner; however, the score was reduced when demonstration was required. The speech assessment in our study includes the repetition of words and sentences, automatic production, and spontaneous speech. In the repetition task, different length words were used, as well as words that require either single or multiple places of articulation (e.g. baby, shoes, shoemaker, “The pretty girl is dancing,” and “The stranger walked along the road”). The automatic production comprises well-established sequences of words such as the months of the year. The spontaneous speech, as suggested by M&O, used the description of the “Cookie Theft” figure from the BDAE (Goodglass and Kaplan, Reference Goodglass and Kaplan1983). For the analysis of speech performance of all M&O tests, the number and types of apraxic manifestations observed in the responses to the M&O protocol were recorded. Patients who had four or more praxis manifestations in the M&O were considered as having speech apraxia. The following types of manifestations that are typical of oral emissions by individuals with apraxia were observed:
° Substitution: replacement of one phoneme by another;
° Omission: default of one phoneme or syllable;
° Addition: insertion of one phoneme or syllable to the word;
° Self-correction: spontaneous correction of apraxic errors;
° Trial and error: sought the articulatory point of a phoneme or sequence of phonemes in a bid to perform the correct movement prior to initiating speech; and
° Repetition: more than one production of sound, word, part of a word, or utterance.
The proportion of errors was calculated according to the number of words spoken. However, because AD patients present speech errors that are also due to language changes caused by dementia, such as the frequent repetition of words and phrases related to the discursive changes (de Lira et al., Reference De Lira, Ortiz, Campanha, Bertolucci and Minett2011), these linguistic errors were not considered during the speech analysis. Only repetitions of segments of words (repetition of sounds or parts of words) were included for measuring apraxic manifestations. Moreover, hesitations during spontaneous speech were disregarded because this characteristic may be present in the speech of healthy individuals and in patients with language or speech disorders.
Patient speech was digitally recorded with a SONY MP3 player and concurrently transcribed. Speech was transcribed to appropriately classify the type of error because trial and error can occur without phonation when the participant seeks the desired phono-articulatory movement for the phoneme without vocalizing.
Statistical methods
We compared our praxis scores with the published means for healthy participants (Radanovic et al., Reference Radanovic, Mansur and Scaff2004) by analyzing the difference between the two means with one-sample Student's t-tests.
To verify if dementia and apraxia severity were significantly related, we performed a multivariate linear regression with apraxia severity as a dependent variable and dementia severity as an independent variable. This analysis was controlled by sex, age, and years of education. The reference group for comparison was the group of patients with mild dementia and the scores obtained from the M&O were analyzed.
A p value of <0.05 was considered statistically significant. All tests were two tailed. All statistical analyses were performed with the SPSS statistical package for Windows, version 17.
Results
General characteristics
Out of the 90 individuals participating in the study, 66 were women. Age varied from 64 to 97 years (mean 80.2±7.2), and the years of education ranged from 0 to 12 years (mean 4.2±3.5). Twelve participants were illiterate, 56 had 1–4 years of schooling, nine had 5–8 years, seven had 9–11 years, and six had over 12 years of schooling; therefore, 76% of patients had less than five years of schooling. The mean MMSE score for those with mild AD was 20.0±3.4, moderate AD was 14.4±1.6, and severe AD was 9.0±2.9. The mean score on the Lawton index for patients with mild AD was 7.0±4.2, with moderate AD was 14.3±2.0, and severe AD was 15.4±1.0.
Descriptive analysis of the praxis scores
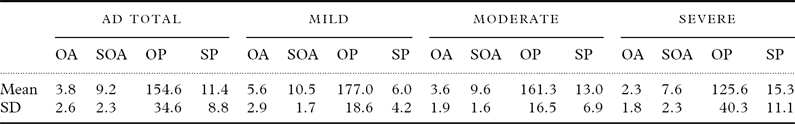
Table 1 shows the mean scores for speech and orofacial praxis tests in the study groups. Seventy-nine patients had orofacial apraxia and 81 had speech apraxia. Only four patients had normal speech and orofacial praxis. Among the 11 patients with normal orofacial praxis, 10 were mild cases of AD and one was of moderate AD. Six out of nine patients without speech apraxia had mild AD and three had moderate AD.
Table 1. Descriptive analysis of the scores on the speech and orofacial praxis tasks by the study group

SD: standard deviation; AD: Alzheimer's disease; OA: orofacial agility on the Boston Diagnostic Aphasia Examination (BDAE; possible range: 0–12, higher scores indicating better performance, normative values: 8.7±2.2); SOA=speech oral agility on the BDAE (possible range: 0–14, higher scores indicating better performance, normative values: 12.1±1.9); OP=orofacial praxis component of the Martins and Ortiz (M&O) protocol (possible range: 0–200, higher scores indicating better performance, and scores below 160 are indicative of orofacial apraxia); SP=speech praxis component of the M&O (the minimum is zero praxis manifestations, higher scores indicating worse performance, and more than three praxis manifestations are indicative of speech apraxia).
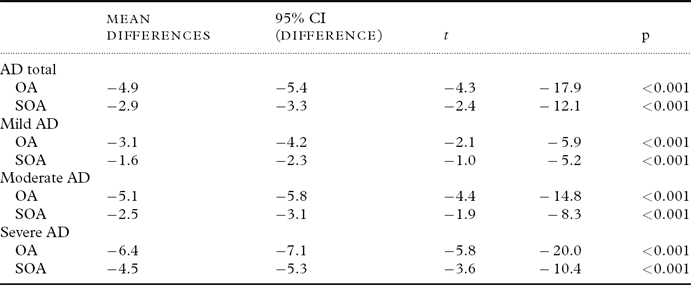
Table 2 shows the differences in the means of orofacial and speech oral agility for patients with AD (mild, moderate, and severe) and the scores estimated for the Brazilian population published by Radanovic et al. (Reference Radanovic, Mansur and Scaff2004). The AD patient means for the oral agility task were significantly lower than the scores estimated for the healthy population.
Table 2. Comparison using Student's t-test for a single sample among the means of the groups with AD and data from the literature on normal populations

AD=Alzheimer's disease; OA=orofacial agility on the Boston Diagnostic Aphasia Examination (BDAE; possible range: 0–12, higher scores indicating better performance, normative values: 8.7±2.2); SOA=speech oral agility on the BDAE (possible range: 0–14, higher scores indicating better performance, normative values: 12.1±1.9).
Association between disease severity and praxis scores controlling for sex, age, and years of education
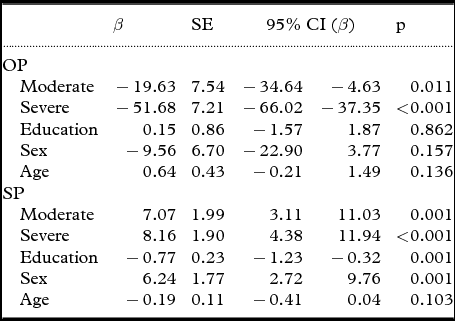
According to the multivariate linear regression analysis using the praxis scores of patients with mild AD as reference group (Table 3), dementia severity was significantly associated with orofacial apraxia severity (moderate AD: β=−19.63, p=0.011; and severe AD: β=−51.68, p < 0.001) and speech apraxia severity (moderate AD: β=7.07, p=0.001; and severe AD: β=8.16, p < 0.001). This analysis was controlled by sex, age, and years of education and revealed that speech praxis scores were influenced by years of education (β=−0.77, p=0.001) and sex (β=6.24, p=0.001), and orofacial praxis scores were not.
Table 3. Multivariate linear regression analyses to verify the association between disease severity and praxis scores (dependent variables) controlling for sex, age, and years of education

SE=standard error; OP=orofacial praxis component of the Martins and Ortiz (M&O) protocol; SP=speech praxis component of the M&O. Reference group: mild stage of AD.
Discussion
The main finding in this study was that speech and orofacial apraxias are nearly always present in AD, regardless of disease stage, even in patients with non-focal disease presentation. Moreover, praxis in patients with AD worsens according to disease stage (severe and moderate < mild).
There are some previously published reports indicating that praxis scores are worse in patients with AD compared with normal individuals (Edwards et al., Reference Edwards, Deuel, Baum and Morris1991; Derouesne et al., Reference Derouesne, Lagha-Pierucci, Thibault, Baudouin-Madec and Lacomblez2000; Crutch et al., Reference Crutch, Rossor and Warrington2007). However, these studies examined ideatory and limb apraxias, and they did not include orofacial and speech praxis assessments. Only a few cases of speech and orofacial apraxias have been described in AD (Kawamura and Mochizuki, Reference Kawamura and Mochizuki1999). This study observed the apraxias during the clinical evaluation of patients with primary progressive speech apraxia; however, AD was diagnosed only after postmortem examination. Furthermore, patient praxic performance was not compared with healthy participant performance. The present study evaluated both types of apraxia in a large sample of patients who were previously diagnosed with probable AD, and patient performance was compared with the mean obtained in a study of healthy Brazilian participants (Radanovic et al., Reference Radanovic, Mansur and Scaff2004). Cera and Ortiz (Reference Cera and Ortiz2009) showed that a language's phonetic–phonological characteristics can affect speakers’ praxic performance and should be considered in data analyses. Cognitive screening must also be analyzed according to what is expected for the population studied. According to Brucki et al. (Reference Brucki, Nitrini, Caramelli, Bertolucci and Okamoto2003), the mean MMSE score for healthy illiterate participants is 19.5 and for minimally educated 24.8. Since in our sample 13% were illiterate and 76% had less than five years of formal education, we expected low MMSE scores even for patients in the early stage of disease. Although our results show that speech praxis scores were influenced by educational level, the praxis scores were significantly associated with disease stage regardless of education level.
Even though working memory is commonly impaired in AD (Belleville et al., Reference Belleville, Peretz and Malenfant1996), its presence does not imply speech apraxia, which is an independent impairment. The pattern of praxic errors observed in the present work was similar to that described in other studies of patients with speech apraxia of other etiologies (Johns and Darley, Reference Johns and Darley1970; Canter et al., Reference Canter, Trost and Burns1985). Therefore, despite the susceptibility to interference from working memory changes on speech praxis performance, the evaluated patients exhibited typical speech apraxia.
In the present study, we observed speech and orofacial apraxias in the same patients. In a study by Ogar et al. (Reference Ogar, Dronkers, Brambati, Miller and Gorno-Tempini2007), orofacial apraxia was present in 11 out of 18 of speech apraxia patients with progressive non-fluent aphasia. Thus, the presence of orofacial apraxia supports the hypothesis that speech manifestations in AD are, in fact, related to apraxia, despite interference from working memory and language. With AD progression, more brain areas are involved and, therefore, more affected cognitive domains are severely compromised. The involvement of areas adjacent to the precentral gyrus of the upper left insula was associated with more severe forms of speech apraxia, as well as the co-occurrence of apraxia and aphasia (Ogar et al., Reference Ogar, Willock, Baldo, Wilkins, Ludy and Dronkers2006). Thus, it is believed that apraxia also worsens with dementia severity.
By correlating speech and orofacial praxis performance with AD severity, we observed that patients with severe and moderate AD performed significantly worse than those in the mild stage of AD (Table 3). As for the relationship between speech apraxia and disease severity, Gerstner et al. (Reference Gerstner, Lazar, Keller, Honig, Lazar and Marshall2007) described speech manifestations in an AD case and showed that they progressed to complete speech loss.
In the present study, patients with severe AD had worse praxic performance than the mild group, and this was characterized by a higher frequency of praxic manifestations. Certain types of apraxia also worsen with dementia severity. Edwards et al. (Reference Edwards, Deuel, Baum and Morris1991) evaluated 142 AD patients and reported that the frequency of patients with ideomotor and ideational apraxia increased with dementia severity. In contrast, Crutch et al. (Reference Crutch, Rossor and Warrington2007) evaluated upper limb praxis and showed that disease severity was not necessarily predictive of the presence of this apraxia. By analyzing the effect of disease severity on the praxic performance of 33 AD patients (15 mild and 18 moderate), these authors found different results depending on the task assessed (e.g. transitive gestures, meaningful intransitive gestures, meaningless intransitive gestures). Thus, they concluded that disease severity only caused interference for some tasks. The likely differences between their results and ours may be the distinction of the apraxia evaluated by Crutch et al. (Reference Crutch, Rossor and Warrington2007), which evaluated upper limb apraxia (not speech apraxia), and also by the sample sizes (33 patients, while this study evaluated 90 individuals).
One possible explanation for the worsening of the speech and orofacial apraxias is that, depending on AD severity, more brain areas are affected, and the general cognitive framework deteriorates.
There are several limitations that should be considered when interpreting the results of this study. The inclusion of patients with severe AD disease stage that were still able to complete the assessment tasks may have contributed to the selection of a sample that minimized differences in speech praxis scores between the moderate and severe stages. Patients in the severe AD group, despite having severe cognitive impairment, had to be able to complete the tests, and for this reason, the mean MMSE score (9.0±2.9) does not correspond to advanced disease stage patients. Out of 103 patients enrolled, five (severe stage) were excluded because they stopped responding to stimuli. Therefore, the standard error of patients with very severe AD may be different from that found in the present study. However, the presence of a severe reduction or suppression of speech prevented the performance of speech analysis and did not allow for a comparison of oral praxic performance among the three disease stages. Thus, the number of errors might have been overestimated in the mild and moderate stages, which would have increased the likelihood of error due to increased response to stimuli. Conversely, patients with severe AD who did not respond to stimuli would show reduced emission and a consequent absence of error. Another limitation of this study was related to the educational level; 76% of our participants had less than five years of formal education. However, we controlled our analyses for education when verifying the association between disease severity and praxis score controlling.
Although there are descriptions of patients who focally present with speech and orofacial apraxias who later receive a diagnosis of AD, we found speech and orofacial apraxias in the early stages of the disease. In summary, speech and orofacial apraxias that can be focal or generalized are frequently present in AD, independent of disease stage. The recognition of early signs of apraxia in AD patients might aid in the evaluation of communication difficulties and potentially in developing rehabilitation strategies.
Conflict of interest
None.
Description of authors’ roles
Maysa Luchesi Cera collected, analyzed, and interpreted the data and wrote the paper. Karin Zazo Ortiz analyzed and interpreted the data, and performed critical revision of the paper. Paulo Henrique Ferreira Bertolucci supervised the data collection and critically revised the paper. Thaís Soares Cianciarullo Minett supervised the collection, analysis and interpretation of the data, and performed critical revision of the paper.