Introduction
Culicoides biting midges (Diptera: Ceratopogonidae) are one of the world's smallest and most abundant blood-sucking flies (Mellor et al., Reference Mellor, Boorman and Baylis2000). Culicoides have a widespread distribution with haematophagous females acting as vectors of various pathogens. Most research on this group focuses on its role in the transmission of viruses (e.g. African horse sickness virus, bluetongue virus or Schmallenberg virus, among others) to livestock, because of its economic impact on the industry (Mellor et al., Reference Mellor, Boorman and Baylis2000; Carpenter et al., Reference Carpenter, Groschup, Garros, Felippe-Bauer and Purse2013; Sick et al., Reference Sick, Beer, Kampen and Wernike2019; Martínez-de la Puente et al., Reference Martínez-de la Puente, Mathieu, Carpenter and Baldet2021). However, Culicoides are well-known vectors of other parasites including avian trypanosomes (Svobodová et al., Reference Svobodová, Dolnik, Čepička and Rádrová2017) and the avian malaria-like Haemoproteus (Valkiūnas, Reference Valkiūnas2005; Martínez-de la Puente et al., Reference Martínez-de la Puente, Martínez, Rivero-de Aguilar, Herrero and Merino2011a), also supporting their role in the transmission of parasites to wildlife. In spite of this, there are still relatively few studies into the ecological interactions between biting midges and wild birds. This is especially relevant considering the impact of Culicoides on the body condition of nestlings (Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008a), together with the deleterious effects of the Culicoides-borne parasites on bird health (Merino et al., Reference Merino, Moreno, Sanz and Arriero2000; Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008a; Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, García-Fraile and Belda2010a). The development of traps inside nest boxes (Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008b; Votýpka et al., Reference Votýpka, Synek and Svobodová2009) allowed researchers to identify the diversity of Culicoides species attracted to bird nests in different European regions (Table 1). For instance, Martínez-de la Puente et al. (Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a) identified the presence of 7 different species of Culicoides in blue tit (Cyanistes caeruleus) nests found in central Spain. Additional studies have also identified which species of Culicoides are attacking birds in their nests, including studies on different avian species conducted in several countries (Czech Republic: Votýpka et al., Reference Votýpka, Synek and Svobodová2009; Spain: Veiga et al., Reference Veiga, Martínez-de la Puente, Václav, Figuerola and Valera2018; Lithuania and Russia: Žiegytė et al., Reference Žiegytė, Platonova, Kinderis, Mukhin, Palinauskas and Bernotienė2021). Moreover, the development of molecular techniques to identify the origin of blood meals from engorged biting midge females has confirmed the ornithophilic feeding preference of most Culicoides species collected from bird nests (Bobeva et al., Reference Bobeva, Zehtindjiev, Ilieva, Dimitrov, Mathis and Bensch2015; Martínez-de la Puente et al., Reference Martínez-de la Puente, Figuerola and Soriguer2015). Thus, the results from these articles support the fact that different species of biting midges are attracted to bird nests, but there is still a lack of studies into what determines Culicoides species composition and abundance.
Table 1. Summary of Culicoides species found in nest boxes of different European bird species, namely blue tit (Cyanistes caeruleus), great tit (Parus major), pied flycatcher (Ficedula hypoleuca) and European roller (Coracias garrulus)

a Votýpka et al. (Reference Votýpka, Synek and Svobodová2009) sampled biting midges from the nests of tree sparrow (Passer montanus), great tit (P. major), blue tit (C. caeruleus), spotted flycatcher (Muscicapa striata), nuthatch (Sitta europaea) and wryneck (Jynx torquilla).
The type of habitat may have a significant effect on the Culicoides species breeding in the area, ultimately determining the abundance and composition of Culicoides inside the bird nests. The type of substrate and water sources are essential for the development of Culicoides larvae (Uslu and Dik, Reference Uslu and Dik2010; Erram et al., Reference Erram, Blosser and Burkett-Cadena2019), but the distance to water sources from avian nests does not seem to affect the abundance of adult biting midges in the nests (Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008b). In addition, weather conditions, which are partially modulated by habitat characteristics, may also influence the abundance of biting midges in avian nests. For example, higher winds early in the morning may negatively affect Culicoides flight performance and, consequently, reduce their abundance in nests (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2009b). Nevertheless, there is scant information about how habitat-dependent variation in environmental factors may affect Culicoides abundance and species composition inside nests. In fact, only a few studies have examined the spatial variation of biting midge abundance in natural habitats. For instance, the management and structure of the habitat may affect the abundance of Culicoides in the area (van Hoesel et al., Reference van Hoesel, Marzal, Magallanes, Santiago-Alarcón, Ibáñez-Bernal and Renner2019), with more Culicoides females captured in the canopy compared to the ground level in a vertical axis (Černý et al., Reference Černý, Votýpka and Svobodová2011). Culicoides abundance may also vary between habitats, but this depends on the host breeding period (Tomás et al., Reference Tomás, Zamora-Muñoz, Martín-Vivaldi, Barón, Ruiz-Castellano and Soler2020) and patch extension (Rivero de Aguilar et al., Reference Rivero-de-Aguilar, Castillo, Moreno, Peñafiel, Browne, Walter, Karubian and Bonaccorso2018). Several factors related to avian host physiology, behaviour and breeding performance may also affect the number of biting midges entering nests. Haematophagous vectors such as Culicoides use different cues (e.g. odourant molecules) to locate their hosts, such as 1-octen-3-ol, carbon dioxide (CO2) and kairomones (Bhasin et al., Reference Bhasin, Mordue (Luntz) and Mordue2000a, Reference Bhasin, Mordue (Luntz) and Mordue2000b; Castaño-Vázquez et al., Reference Castaño-Vázquez, Merino, Cuezva and Sánchez-Moral2020). This may at least partly explain the positive correlation between Culicoides abundance and brood size in different bird species (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a, Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2009b; Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Lobato, Rivero-De Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2010b; Castaño-Vázquez and Merino, Reference Castaño-Vázquez and Merino2021), likely due to a greater release of attractive molecules from nests with larger broods.
The aim of this study was to identify the effect of habitat type on the abundance and species composition of biting midge Culicoides attacking avian hosts in their nests. To this end, we sampled Culicoides in nest boxes occupied by blue tits in 2 neighbouring forests in southern Spain with different environmental characteristics during the bird's breeding season for 3 consecutive years. We first compared the prevalence, abundance and species richness in nests situated in the 2 habitat types over the first 2 years. Secondly, we developed a cross-fostering experiment based on the results obtained and considering that the observed differences between the 2 forests could be due to the effects of habitat type on the emission of nestling cues (e.g. microbiota, Ruiz-López, Reference Ruiz-López2020; or the composition of uropygial secretions, Tomás et al., Reference Tomás, Zamora-Muñoz, Martín-Vivaldi, Barón, Ruiz-Castellano and Soler2020), rather than a direct association between habitat type and Culicoides. This study design meant we could examine the role of rearing environment conditions (i.e. forest type) independently from the genetically determined attractants released by birds in each forest.
Materials and methods
Study area
The study was carried out during the spring of 2017, 2018 and 2019 using blue tits breeding in nest boxes in the Sierra Nevada National Park (southeast Spain, 36°57′N, 3°24′W, 1700–1800 m a.s.l.). Nest boxes were placed in 2 different, but adjacent forests separated by approximately 1.5 km. One site was a dry Pyrenean oak (Quercus pyrenaica) deciduous forest (hereinafter, ‘dry forest’), while the other was a mixed forest consisting mainly of Pyrenean oaks along with some Holm oaks (Quercus ilex) and which was crossed by a stream (Acequia Almiar), conferring it a moister ambient (hereinafter, ‘humid forest’). The humid forest had a higher relative humidity, lower mean temperature, higher solar irradiation and lower insolation time compared to the dry forest (see Supplementary material; Garrido-Bautista et al., Reference Garrido-Bautista, Soria, Trenzado, Pérez-Jiménez, Ros-Santaella, Pintus, Bernardo, Comas and Moreno-Rueda2021).
Blue tit sampling and cross-fostering experiment
Overall, 199 nest boxes were occupied by blue tits over the 3 years (69 in 2017, 56 in 2018 and 74 in 2019), of which 95 were randomly selected and followed to collect the data included in this study. Biting midges were monitored in 45 nest boxes in the dry forest and 50 in the humid forest. All the nest boxes were ICONA C model (details in Moreno-Rueda, Reference Moreno-Rueda2003) and were cleaned every year before the breeding season. They were hung from an oak tree's branch at a height of 3–4 m. We monitored the nest boxes each year to determine the hatching date (day the first egg hatched each year = day 0) and brood size at day 13.
During the spring of 2019, we conducted a cross-fostering study to identify the potential effect of nestling characteristics in the 2 habitats on Culicoides abundance in their nests. Hence, we designed a cross-fostering experiment in which whole broods were exchanged between the dry and humid forests or within each forest, according to the treatment. When nestlings were 3 days old, broods of the same age were exchanged according to their size (±2 nestlings) using warm, breathable bags. Nestling broods exchanged and reared in the same forest served as manipulation controls. All nestlings in the nest boxes were exchanged, but the procedure was performed in 2 steps to ensure the nests always contained at least 3 nestlings and therefore prevent parent desertion. In total, the cross-fostering experiment included broods from 35 nests: 11 broods were exchanged within the humid forest (humid–humid treatment), 6 within the dry forest (dry–dry treatment), 9 were moved from the dry to the humid forest (dry–humid treatment) and 9 were moved from the humid to the dry forest (humid–dry treatment).
Culicoides collection and identification
Biting midges were captured in blue tit nest boxes following the method described by Tomás et al. (Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008b) with minor modifications. A Petri dish (60 mm diameter) layered with body gel-oil (Johnson's® Baby Oil Gel with Chamomile, Johnson and Johnson, Dusseldorf, Germany) was placed in the inner roof of the nest boxes when nestlings were 12 days old. The gel-oil was made up of paraffinum liquidum, hexyl laurate, ethylene/propylene/styrene copolymer, cyclopentasiloxane, butylene/ethylene/styrene copolymer, chamomilla recutita, bisabolol and perfume (FPT1353). The Petri dishes were collected the next day, when nestlings were 13 days old, and stored in a freezer until further analysis.
The biting midges were removed from the Petri dishes by applying xylene for a few seconds, then passed to absolute ethanol. After approximately 5 min, the biting midges were transferred to Eppendorf tubes with 70% ethanol and maintained at −20°C. The Culicoides specimens were sexed and identified to the species level according to their morphological characteristics (e.g. wing spot patterns and the presence of coeloconic sensilla on the antennae) and based on available keys (Rawlings, Reference Rawlings1996; González and Goldarazena, Reference González and Goldarazena2011), including the IIKC website (Mathieu et al., Reference Mathieu, Cêtre-Sossah, Garros, Chavernac, Balenghien, Carpenter, Setier-Rio, Vignes-Lebbe, Ung, Candolfi and Delécolle2012). Species identification was further confirmed by mounting between 4 and 10 individuals of each species. The parity of Culicoides females was determined visually: (1) those that had never fed on blood (nulliparous females), (2) those showing a burgundy pigment in the subcutaneous cells of the abdomen indicating a previously digested blood meal (parous females; Dyce, Reference Dyce1969) and (3) those with a recent blood meal in their abdomen (engorged females). We calculated the species richness for each nest box as the sum of the different Culicoides species collected. The prevalence of biting midges was calculated as the percentage of infested nests with respect to the total number of nests analysed. We estimated the abundance of Culicoides as the number of specimens captured for all the species in each nest. The total abundance of Culicoides was calculated as the sum of nulliparous, parous and engorged females per nest, while also considering any unidentified individuals.
Statistical analyses
We used Cleveland plots to check for outliers in the abundance of biting midges and tested the normality of the abundances of Culicoides and species richness graphically (Zuur et al., Reference Zuur, Ieno and Elphick2010). An outlier was detected in a nest box from the humid forest in 2019, which far exceeded the standard deviation (s.d.) of the mean biting midge abundance (mean ± s.d. = 1.04 ± 1.73; n = 94; outlier: 49 individuals). This outlier probably reflected a close breeding area of Culicoides reconditus, as 39 out of 49 of the individuals collected corresponded to this species, and 32 of the 39 were nulliparous females. Thus, we performed the analyses using both the original dataset and one that excluded the outlier. Models including the outlier gave qualitatively the same results as those without it. Here, we report the statistical analyses without the outlier, although we included it in the descriptive statistics. The total abundance of Culicoides females and species richness followed a Poisson distribution. Analyses on Culicoides species were restricted to the 2 most common species captured, namely C. reconditus and Culicoides circumscriptus (see Table 2).
Table 2. Abundance of Culicoides species captured in blue tit nests from 2 different types of forests during the breeding seasons of 2017, 2018 and 2019

The percentage of infected nests is shown in parentheses.
Generalized linear mixed models (GLMMs) with a Poisson distribution and a logit-link function were used to examine the variation in the abundance of biting midges and species richness with forest type. For data collected in 2017 and 2018, the full models had the following structure: total abundance, abundance of C. reconditus, abundance of C. circumscriptus and species richness were included as dependent variables in separate models; forest type, year and their interaction were fixed factors; hatching date and day 13 brood size were covariates and nest identity was a random factor. We decided to incorporate hatching date and brood size in the models as previous studies highlighted their importance when interpreting the abundance of biting midges in blue tit nests (Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008a; Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a, Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2009b; Castaño-Vázquez and Merino, Reference Castaño-Vázquez and Merino2021). We did not consider the impact of the forest–year interaction on the abundance of C. circumscriptus because no specimens were collected in the humid forest in 2017. GLMM with a binomial distribution and a logit-link function was used to test the relationship between the presence/absence of Culicoides in nests and the type of forest. The presence/absence of Culicoides was used as a dependent variable, and forest, year and forest–year interaction as fixed factors. Hatching date and brood size were introduced as covariates and nest identity as random factor.
Generalized linear model (GLM) with a Poisson distribution and a logit-link function was used to test the effect of the cross-fostering experiment. In this case, total Culicoides abundance and species richness were included as the dependent variables in each full model and forest of origin, forest of fostering and their interaction were included as fixed factors, with hatching date and brood size as covariates. A full GLM, with a binomial distribution and a logit-link function, and the same structure as the cross-fostering experiment, was also used to check the relationship between the presence/absence of biting midges and treatments.
In all cases, we applied a model–selection approach to choose the best models of all possibilities derived from the aforementioned full models. To do so, we used Akaike's information criterion (AIC) and selected models within a ΔAIC <2 units (Quinn and Keough, Reference Quinn and Keough2002). The parameters were estimated by model averaging all models with a ΔAIC under 2 units (Symonds and Moussalli, Reference Symonds and Moussalli2011). We tested the normality of the residuals from the models graphically following Zuur et al. (Reference Zuur, Ieno and Elphick2010). For the descriptive analyses, we used the Pearson product–moment correlation to examine the correlations between continuous variables. A t-test was used to analyse the differences in hatching dates between infected and uninfected nests. The basic statistics are given as mean ± standard error (s.e.). All analyses were performed in software R v 4.0.0 (R Development Core Team, 2020), using the packages ‘lme4’ (Bates et al., Reference Bates, Maechler, Bolker, Walker, Christensen, Singmann, Dai, Scheipl, Grothendieck, Green and Fox2020) and ‘MuMIn’ (Bartoń, Reference Bartoń2020).
Results
A total of 147 female biting midges were captured in 42 of the 95 nests monitored during the 3 years (prevalence 44.21%), corresponding to 5 different species (Table 2). Four biting midges (2.72% of the total captured) were not identified to the species level because they lacked wings or other distinctive structures. A mean of 1.55 ± 0.54 (range: 0–49) biting midges were captured per nest. In nests with biting midges, there was a mean of 1.57 ± 0.13 different species (range: 1–4) per nest. Culicoides reconditus (40.14%) and C. circumscriptus (22.45%) were the most common species found in blue tit nests (Table 2). Most of the 147 Culicoides females captured were nulliparous (75.55%), while we captured 34 parous females (23.13%) and only 2 engorged females (1.36%). Parous females corresponded to the species C. circumscriptus (n = 14), C. reconditus (n = 11), Culicoides truncorum (n = 4), Culicoides kibunensis (n = 2), Culicoides festivipennis (n = 1) and 2 parous individuals were not identified to the species level. The engorged females belonged to the species C. truncorum and C. kibunensis. No males were found in the nests.
Correlative analyses
Table 3 provides a summary of the results of the model selection for the correlative study. The best model for Culicoides abundance included forest and hatching date as predictor variables, while the second-best model also included the year (although it was not significant). The abundance of Culicoides captured in blue tit nests was higher in the dry (1.60 ± 0.41) than in the humid forest (0.63 ± 0.21; estimate = 0.99, z = 2.31, P = 0.021; Fig. 1A) and correlated positively with hatching date (estimate = 0.12, z = 3.50, P < 0.001; r = 0.32, P = 0.014; Fig. 2A). The best model for the abundance of C. circumscriptus included forest type, year and hatching date as predictors, and the second best also included brood size as a predictor, although it was not significant. The same forest-dependent variation was found for the abundance of C. circumscriptus (estimate = 1.25, z = 2.07, P = 0.039; Fig. 1B), which was higher in 2018 than in 2017 (estimate = 1.94, z = 2.37, P = 0.018; Fig. 1B). In addition, the abundance of C. circumscriptus increased when the nestlings were hatched later in the season (estimate = 0.10, z = 1.96, P = 0.049; r = 0.27, P = 0.039; Fig. 2B). The model selected for the abundance of C. reconditus included brood size and hatching date as predictors. We found a negative and significant relationship between the abundance of C. reconditus and brood size (χ 2 = 8.94, P = 0.003; r = −0.29, P = 0.024). The abundance of C. reconditus was positive and significantly related to hatching date (χ 2 = 6.01, P = 0.014; Fig. 2B). With respect to species richness, the best model included hatching date and brood size as predictors, but only hatching date was significant. Three additional models had a ΔAIC <2, but only hatching date had a significant effect on species richness in all these models. Specifically, species richness was positively associated with hatching date (estimate = 0.09, z = 3.02, P = 0.002; r = 0.31, P = 0.016; Fig. 2C). Finally, the best models for the presence/absence of biting midges included hatching date, forest and year as predictors, but only hatching date significantly affected the presence of Culicoides in all these models. The presence of Culicoides correlated positively with hatching date (estimate = 0.17, z = 2.65, P = 0.008), suggesting that nests with late-hatching nestlings were more likely to be infected by biting midges than those which hatch earlier in the breeding season (t-test: t 58 = −2.75, P = 0.008).

Fig. 1. Abundance of biting midges (Culicoides) (A), abundance of Culicoides circumscriptus (B), abundance of Culicoides reconditus (C) and species richness (D) in blue tit nests located in humid and dry forests during the breeding seasons of 2017 and 2018. Means were calculated without the outlier. Bars represent s.e.
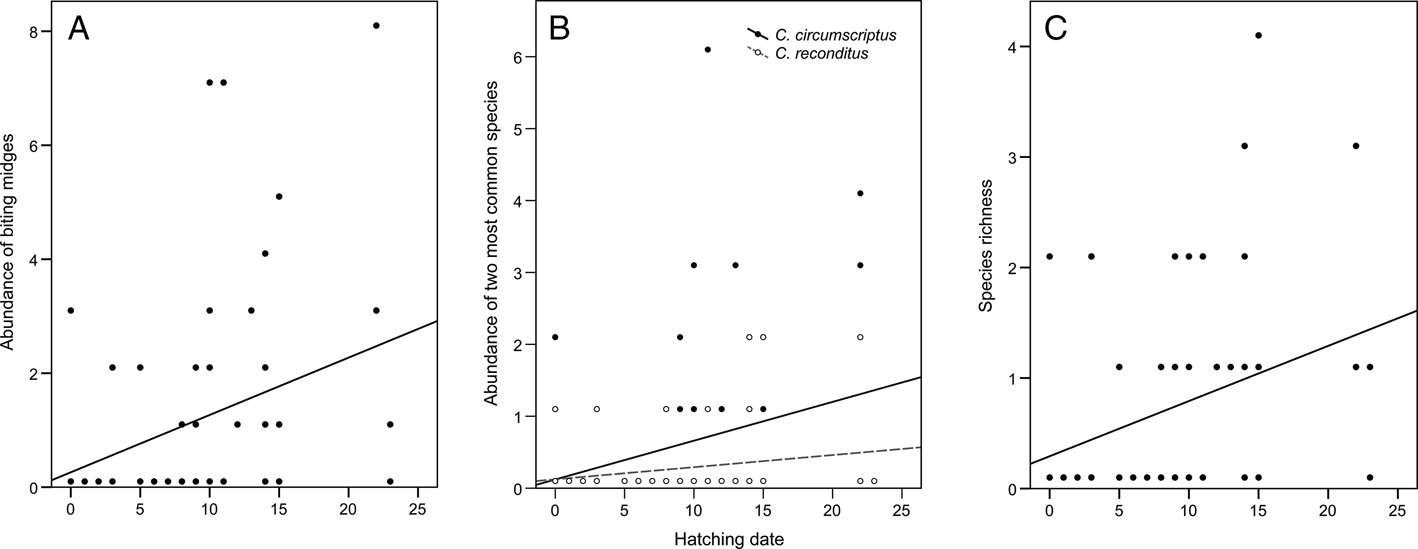
Fig. 2. Relationships between hatching date and the abundance of biting midges (Culicoides) (A), the abundance of the 2 most common species (C. reconditus and C. circumscriptus) (B) and species richness (C) in blue tit nests during the breeding seasons of 2017 and 2018. The regression lines were calculated without the outlier (total abundance: adjusted R 2 = 0.084, P = 0.014; abundance of C. reconditus: R 2 = 0.021, P = 0.137; abundance of C. circumscriptus: R 2 = 0.055, P = 0.039; species richness: adjusted R 2 = 0.081, P = 0.016). The hatching date is standardized (0 = day the first egg hatched each year).
Table 3. Models (within ΔAIC <2 units) describing the total abundance of Culicoides, species richness, biting midge prevalence and the abundance of the 2 most common Culicoides species (C. reconditus and C. circumscriptus) in blue tit nests during the breeding seasons of 2017 and 2018

The significant predictors (P < 0.05) are marked in bold.
Cross-fostering experiment
Table 4 shows the model selection results for the cross-fostering experiment conducted in 2019. The results reflect that the independent variables did not have a significant effect on either species richness or biting midge prevalence as none of the models differed significantly from the null models (Table 4). However, 2 different models for Culicoides abundance had a ΔAIC <2 each including hatching date and foster forest (model 1) and hatching date, foster forest and origin forests (model 2) as predictors. However, only hatching date and foster forest had a significant impact on Culicoides abundance. As for the correlative study, the abundance of Culicoides was higher in the dry (1.33 ± 0.52) than in the humid forest (0.58 ± 0.19; estimate = 0.85, z = 2.13, P = 0.033) and was positively associated with hatching date (estimate = 0.11, z = 2.70, P = 0.007).
Table 4. Models (within ΔAIC <2 units) describing the total abundance of Culicoides, species richness and prevalence of biting midges in blue tit nests during the cross-fostering experiment conducted in 2019

The significant predictors (P < 0.05) are marked in bold.
a Null models only include the intercept.
Discussion
We identified Culicoides species composition and examined different factors that influence their abundance in blue tit nests. We found 5 Culicoides species, all previously captured in bird nest boxes in other regions (Table 1). In addition, 3 out of 5 Culicoides species found here were previously recorded in nests from the same bird species in central Spain, although the dominant species clearly differed between locations (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a; this study). Specifically, Culicoides simulator was the most common species in central Spain accounting for 56.89% of all Culicoides captured in blue tit nests, while the most common species observed in the present study was C. reconditus (40.14%). Interestingly, to the best of our knowledge, this is the first account of C. reconditus in the Iberian Peninsula (Delécolle, Reference Delécolle and Carles-Tolrá Hjorth-Andersen2002; Alarcón-Elbal and Lucientes, Reference Alarcón-Elbal and Lucientes2012). In a previous study in central Spain, Martínez-de la Puente et al. (Reference Martínez-de la Puente, Martínez, Rivero-de Aguilar, Herrero and Merino2011a) found a high intraspecific genetic variance of a fragment of the cytochrome c oxidase subunit 1 gene in specimens morphologically identified as Culicoides segnis, which suggests the sequences could correspond to 2 different species. The authors argued that the results could be due to the presence of C. reconditus in the area, because it is closely related to C. segnis only differing in the distribution of their coeloconic sensilla on the antennae and shape of abdominal sclerites (Mathieu et al., Reference Mathieu, Cêtre-Sossah, Garros, Chavernac, Balenghien, Carpenter, Setier-Rio, Vignes-Lebbe, Ung, Candolfi and Delécolle2012). These characteristics were clearly identified in 10 specimens mounted in this study, 2 of which were deposited in the National Museum of Natural Sciences (MNCN-CSIC), Madrid, Spain, mounted on 4 slides (2 per individual) under the accession numbers MNCN_Ent_319173, MNCN_Ent_267740, MNCN_Ent_267741 and MNCN_Ent_267742.
Correlative analyses
We found habitat influenced on the abundance of biting midges with a higher abundance in nests located in the dry forest compared to the humid forest over the 2 years of the correlative study. Some of the possible causes of this difference include the interhabitat variation in the total abundance of Culicoides and/or because the Culicoides have a different capacity to reach avian nests in each habitat. The availability and extension of water sources in a habitat, together with abiotic soil characteristics, are strong determinants of Culicoides abundance because of their importance for larval development (Uslu and Dik, Reference Uslu and Dik2010; Erram et al., Reference Erram, Blosser and Burkett-Cadena2019). For instance, C. festivipennis preferably breeds in nutrient-rich muds found in streams, while other species, such as C. circumscriptus, are more generalist when breeding (Uslu and Dik, Reference Uslu and Dik2010, and references therein). On the other hand, the weather conditions in the 2 habitats are evidently different; the dry forest had a higher temperature and a lower humidity than the humid forest (Supplementary material; Garrido-Bautista et al., Reference Garrido-Bautista, Soria, Trenzado, Pérez-Jiménez, Ros-Santaella, Pintus, Bernardo, Comas and Moreno-Rueda2021). The relative humidity may negatively affect the large-scale abundance of biting midges in the area (van Hoesel et al., Reference van Hoesel, Marzal, Magallanes, Santiago-Alarcón, Ibáñez-Bernal and Renner2019), which could partially explain the lower Culicoides abundance in the humid forest. In addition, in an experimental study affecting the humidity inside nest boxes occupied by European roller (Coracias garrulus), Castaño-Vázquez et al. (Reference Castaño-Vázquez, Merino, Valera and Veiga2022) found a lower abundance of Culicoides in nests with a higher humidity. Nevertheless, humidity seems to be less determinant for Culicoides abundance and flight performance than temperature. During the breeding season of the blue tits, ambient temperature correlates with nest temperature (Ardia et al., Reference Ardia, Pérez and Clotfelter2006), while Culicoides abundance increases with ambient temperature (Bernotienė et al., Reference Bernotienė, Bartkevičienė and Bukauskaitė2021; Castaño-Vázquez and Merino, Reference Castaño-Vázquez and Merino2021) and temperature inside the nest (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Lobato, Rivero-De Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2010b). In fact, heat gradients are important cues which biting midges, and other vectors, use to locate their hosts (Lehane, Reference Lehane2005).
On the other hand, other variables, such as early morning wind speed – when biting midges are more active – (Lehane, Reference Lehane2005), could affect the number of vectors visiting nest boxes. The dry and humid forests were located opposite each other on south-west and south-east mountain slopes, respectively. Since the prevailing wind in this region is westerly during spring (Viedma-Muñoz, Reference Viedma-Muñoz1998), the humid forest was expected to receive higher wind speeds, ultimately reducing Culicoides flight activity and, consequently, decreasing their abundance in bird nests (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2009b). Variations in forest leaf density between large areas may also impact Culicoides population numbers, with some species favouring sparsely vegetated areas, while others prefer habitats with a higher leaf density (Conte et al., Reference Conte, Goffredo, Ippoliti and Meiswinkel2007). This variation in forest cover goes some way to explaining the different abundances of some Culicoides species between habitats. Lastly, we should not ignore the fact that the interforest variation in Culicoides abundance could be due to a geographical singularity of the 2 sampled localities independent of the habitat differences; however, unfortunately, we cannot study this premise as we do not have any spatial replicates for this system.
Differences in the weather conditions may also explain the positive association between hatching date and all the variables analysed. This implies that nestlings from nests breeding later in the season were affected by more Culicoides and from more species. As the ambient temperature increases throughout the breeding season, we would normally expect more biting midges to visit more nests (e.g. Bernotienė et al., Reference Bernotienė, Bartkevičienė and Bukauskaitė2021). Several studies have reported effects of seasonality on the abundance of different vector groups, including Culicoides, and found that their abundance generally augmented as the spring progressed (Sarto i Monteys and Saiz-Ardanaz, Reference Sarto i Monteys and Saiz-Ardanaz2003; Ferraguti et al., Reference Ferraguti, Martínez-de la Puente, Muñoz, Roiz, Ruiz, Soriguer and Figuerola2013; Lalubin et al., Reference Lalubin, Delédevant, Glaizot and Christe2013; Bernotienė et al., Reference Bernotienė, Bartkevičienė and Bukauskaitė2021). This was also true of cavity-nesting birds, as Culicoides abundance in their nests increased as the breeding season advanced (Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008a; Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a, Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2009b; Castaño-Vázquez and Merino, Reference Castaño-Vázquez and Merino2021; this study). In addition to the detrimental effect of the blood-sucking activity of Culicoides, these results support the fact that nestlings which hatch later in the season may be subject to a greater susceptibility to blood parasite infections (Martínez-de la Puente et al., Reference Martínez-de la Puente, Martínez, Rivero-de-Aguilar, del Cerro and Merino2013). At least, 4 out of 5 species of Culicoides captured here may act as vectors for Haemoproteus parasites, and parous females of all of these species have been found in avian nests (C. circumscriptus: Martínez-de la Puente et al., Reference Martínez-de la Puente, Martínez, Rivero-de Aguilar, Herrero and Merino2011a; Veiga et al., Reference Veiga, Martínez-de la Puente, Václav, Figuerola and Valera2018; C. festivipennis: Martínez-de la Puente et al., Reference Martínez-de la Puente, Martínez, Rivero-de Aguilar, Herrero and Merino2011a; C. kibunensis: Martínez-de la Puente et al., Reference Martínez-de la Puente, Martínez, Rivero-de Aguilar, Herrero and Merino2011a; Bernotienė et al., Reference Bernotienė, Žiegytė, Vaitkutė and Valkiūnas2019; Žiegytė et al., Reference Žiegytė, Platonova, Kinderis, Mukhin, Palinauskas and Bernotienė2021; C. truncorum: Martínez-de la Puente et al., Reference Martínez-de la Puente, Martínez, Rivero-de Aguilar, Herrero and Merino2011a). Thus, nestlings from late-nesting parents could be impaired in terms of future reproduction success (Merino et al., Reference Merino, Moreno, Sanz and Arriero2000) or even long-term survival (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, García-Fraile and Belda2010a). Further studies should be conducted in order to identify the parasites potentially transmitted by Culicoides species in the area.
Furthermore, there was a negative relationship between insect abundance and brood size in the case of C. reconditus, while the other variables analysed returned statistically non-significant associations. If more nestlings release a greater concentration of attractive molecules (e.g. CO2, kairomones), then one would expect higher Culicoides abundances in nests with larger broods (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a, Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2010b; Castaño-Vázquez and Merino, Reference Castaño-Vázquez and Merino2021). Nevertheless, contrasting results have previously been reported in blue tits, with some studies showing a positive relationship between Culicoides abundance and brood size (Martínez-de la Puente et al., Reference Martínez-de la Puente, Merino, Tomás, Moreno, Morales, Lobato, Talavera and Sarto i Monteys2009a, Reference Martínez-de la Puente, Merino, Lobato, Rivero-de Aguilar, del Cerro, Ruiz-de-Castañeda and Moreno2009b), yet another study also reported non-significant associations (Tomás et al., Reference Tomás, Merino, Martínez-de la Puente, Moreno, Morales and Lobato2008a). Given the correlative nature of these results, further experimental research into different brood sizes is required to clarify the influence of brood size on the birds' susceptibility to Culicoides attacks.
Cross-fostering experiment
Based on the results from the previous 2 years, we developed an experimental approach to identify the role of habitat vs nestling traits in determining the birds' susceptibility to Culicoides attacks. Nestlings from different habitats could produce different attractants to insect vectors. For example, different studies have proposed that odours derived from avian uropygial gland secretions are involved in the attraction of different vectors (Russell and Hunter, Reference Russell and Hunter2005; Garvin et al., Reference Garvin, Austin, Stracker, Slowinski, Rutter, Butler, Michel and Whelan2018; review in Moreno-Rueda, Reference Moreno-Rueda2017; Tomás et al., Reference Tomás, Zamora-Muñoz, Martín-Vivaldi, Barón, Ruiz-Castellano and Soler2020). This secretion, together with other scent-producing body sources, such as the skin or feathers (Menon and Menon, Reference Menon and Menon2000; Campagna et al., Reference Campagna, Mardon, Celerier and Bonadonna2012), may determine bird odour (Campagna et al., Reference Campagna, Mardon, Celerier and Bonadonna2012) and probably has a genetic origin (Krause et al., Reference Krause, Bischof, Engel, Golüke, Maraci, Mayer, Sauer and Caspers2018). For the case of biting midges, Tomás et al. (Reference Tomás, Zamora-Muñoz, Martín-Vivaldi, Barón, Ruiz-Castellano and Soler2020) found that uropygial secretions from hoopoe (Upupa epops) nestlings may repel some insect vectors, depending on the habitat. However, other studies failed to identify any similar associations in biting midges (Martínez-de la Puente et al., Reference Martínez-de la Puente, Rivero-de Aguilar, del Cerro, Argüello and Merino2011b) and mosquitos (Díez-Fernández et al., Reference Díez-Fernández, Martínez-de la Puente, Gangoso, Ferraguti, Soriguer and Figuerola2019). In addition, uropygial secretions can harbour symbiotic bacteria that may release several chemical cues (Maraci et al., Reference Maraci, Engel and Caspers2018), which, together with the skin microbiota, may affect vector attraction (reviewed in Ruiz-López, Reference Ruiz-López2020). However, in this work we failed to identify any significant association supporting this scenario, which suggests that habitat may have a major significant impact on nestling exposure to biting midges. Our results indicate that the habitat of origin of blue tit nestlings did not affect the degree to which they attracted biting midges to their nests, suggesting that nestlings did not exhibit repellent or attractant chemical properties to these vectors unrelated to rearing habitat (due to genetic differentiation or maternal effects). On the other hand, the abundance of biting midges did differ between habitats, lending further support to the influence of this variable on Culicoides abundance in avian nests.
Concluding remarks
The abundance of biting midges in blue tit nests is mainly determined by habitat type, which may explain the different patterns of blood–parasite transmission observed in birds from different habitats (e.g. Ferraguti et al., Reference Ferraguti, Martínez-de la Puente, Bensch, Roiz, Ruiz, Viana, Soriguer and Figuerola2018). It is important to take these results into account when trying to understand local variations in bird species' susceptibility to vector attacks. These attacks may adversely affect the health and fitness of wild birds due to the detrimental effects of their bites and the parasites they can transmit. This should be especially relevant due to the impact of global change on the distribution and abundance of vectors of pathogens, including those attacking birds in their nests (Castaño-Vázquez and Merino, Reference Castaño-Vázquez and Merino2021).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118202200066X.
Data availability statement
Data will be provided by the authors under reasonable request.
Acknowledgements
We are grateful to staff at Sierra Nevada National Park for their support. We also wish to acknowledge the Entomology Department of the National Museum of Natural Sciences (MNCN-CSIC), Madrid, Spain, for cataloguing the Culicoides reconditus specimens in its collection. Abelardo Requena Blanco assisted us during the fieldwork in 2018, and Carmen Hernández Ruiz helped with GIS analyses included in the Supplementary material. The comments from 2 anonymous referees significantly improved the quality of the manuscript.
Author contributions
J. M. P. and G. M. R. conceived and designed the study. J. G. B., J. L. R.-S., E. P., P. L., N. B., M. C. and G. M. R. collected the field data. J. G. B. and J. M. P. identified the Culicoides species and developed the statistical analyses. J. G. B. wrote the first draft of the manuscript with input from the other authors. J. M. P. and G. M. R. secured the funding. G. M. R. coordinated and supervised the project. All authors read and approved the final version of the manuscript.
Financial support
This study was partially funded by projects within the National Plan of the Spanish Ministry of Economy and Competition (CGL2014-55969-P and CGL2017-84938-P), the Spanish Ministry of Science and Innovation (PID2020-118205GB-I00) and the Andalusian government (A.RNM.48.UGR20), co-funded with FEDER funds from the European Union. J. L. R.-S. and E. P. were supported by Erasmus+ grants from the European Union. J. G. B. was supported by an FPU predoctoral contract from the Spanish Ministry of Education (FPU18/03034). Funding for open access charge: Universidad de Granada.
Conflict of interest
The authors declare there are no conflicts of interest.