Clinical implications
-
• Anyone prescribed a dopamine-receptor blocking agent for whatever reason and sustained length of time is at risk of developing potentially irreversible tardive dyskinesia manifested by abnormal involuntary movements that range in phenomenology, severity, and impact.
-
• Tardive dyskinesia can range from subtle movements in one body area that may be mildly embarrassing and resemble common mannerisms to painful, disfiguring, or generalized movements that are stigmatizing and incapacitating.
-
• A rational strategy for management includes screening at all clinical visits, documenting abnormal movements, discussion of treatment options with patients and caregivers, modification of psychotropic medications, and consideration of specific antidyskinetic treatment with vesicular monoamine transporter inhibitors.
Introduction
In 1957, a few years after chlorpromazine was first given to patients with psychosis,Reference Delay, Deniker and Harl 1 Schonecker et alReference Schonecker 2 reported 3 elderly women who developed persistent dyskinesias after treatment. Subsequent observations confirmed the occurrence of a complex phenomenological picture of abnormal movements that were distinguished from previously described drug-induced movements by a delayed onset and persistence after antipsychotics were discontinued.Reference Sigwald, Bouttier, Raymondeau and Piot 3 -Reference Owens 6
Despite initial resistance, the significance of tardive dyskinesia (TD) became a major impetus for developing drugs effective against psychotic symptoms without affecting motor circuitry.Reference Caroff, Ungvari and Cunningham Owens 7 While extensive research by many prominent groups established the clinical and epidemiological dimensions of TD,Reference Klawans 8 -Reference Correll, Kane and Citrome 18 the landmark study of clozapine by Kane et alReference Kane, Honigfeld, Singer and Meltzer 19 served as a proof-of-concept for industry stakeholders in developing new drugs that preserved and extended antipsychotic efficacy while reducing the risk of TD and other drug-induced movement disorders. Paradoxically, success of the subsequent new antipsychotics extended risk of TD to more patients as the proliferation of new drugs, indications, and off-label use exploded. While awareness of TD waned among psychiatrists,Reference Kane 20 , Reference Meyer 21 TD continued to occur and remained a concern among movement disorder specialists.Reference Jankovic and Clarence-Smith 22 -Reference Hauser and Truong 25
More recently, industry-sponsored clinical trials led to the approval of two novel vesicular monoamine transporter-2 (VMAT2) inhibitors, valbenazine and deutetrabenazine,Reference O’Brien, Jimenez and Hauser 26 -Reference Citrome 31 with more favorable pharmacokinetic properties than tetrabenazine which had long been recognized as effective in suppressing TD movements.Reference Jankovic and Clarence-Smith 22 , Reference Brandrup 32 -Reference Kenney and Jankovic 34 Availability of these evidence-based treatments prompted renewed industry support for research on TD. We sought to briefly summarize new evidence for detecting, diagnosing, and treating TD.
Identifying and stratifying risk for TD during routine examination
Incidence and prevalence
A guiding principle for antipsychotic treatment is that anyone prescribed a dopamine-receptor blocking agent for whatever reason and sustained length of time is at risk for developing potentially irreversible TD manifested by abnormal involuntary movements that range in phenomenology, severity, and impact. TD is not uncommon. In general, the incidence of new cases of TD is reported to be approximately 3% to 5% annually, rising cumulatively at this rate for at least the first 3–5 years of antipsychotic treatment.Reference Kane, Bloom and Kupfer 35 -Reference Toenniessen, Casey and McFarland 39 However, annualized rates of TD should not be misinterpreted as meaning TD occurs only after exposure of a year or more, as TD may occur within a few weeks in vulnerable patients.Reference Schonecker 2 , Reference Caroff, Citrome and Meyer 40 -Reference Jeste, Lacro, Bailey, Rockwell, Harris and Caligiuri 43 The prevalence of TD in populations of treated patients is reported to average approximately 20% to 30%.Reference Kane, Bloom and Kupfer 35 , Reference Carbon, Hsieh, Kane and Correll 36
Risk factors
Keeping the dictum of universal vulnerability in mind, there are varying degrees of risk to consider. Advancing age is the strongest predictor of TD, such that antipsychotics should be prescribed cautiously in people 45 years or older.Reference Jeste 44 -Reference Lieberman, Kane, Woerner and Weinhold 48 The incidence and prevalence of TD among older adults may reach 15% to 30% annually and 50% to 60%, respectively.Reference Jeste 44 , Reference Saltz, Woerner and Kane 49 Sex, race, and ethnicity are less consistent risk factors as contrasting prescribing practices affecting demographic groups may be one of many confounding variables.Reference Miller, McEvoy and Davis 45 -Reference Patterson-Lomba, Ayyagari and Carroll 47 , Reference van Harten, Matroos, Hoek and Kahn 50 -Reference Kumsa, Girma, Alemu and Agenagnew 53 Schizophrenia, which like aging predisposes to spontaneous dyskinesias,Reference Caroff, Ungvari and Cunningham Owens 7 , Reference Khot and Wyatt 54 -Reference Caroff, Leong, Roberts, Berkowitz and Campbell 58 confers a high risk of TD. Severity of positive, negative, and cognitive symptoms has been associated independently with TD,Reference Miller, McEvoy and Davis 45 , Reference Solmi, Pigato, Kane and Correll 46 , Reference Caroff, Davis and Miller 59 although confounded by higher dosing and longer use of potent agents with frequent noncompliance in patients with more severe symptoms of chronic schizophrenia. Developmental disorders and mood disorders, primarily bipolar disorder, also may confer risk.Reference Caroff, Mu, Ayyagari, Schilling, Abler and Carroll 60 -Reference Casey 62 But more women and older patients with depression who receive intermittent treatment with antipsychotics during episodic relapses may confound the association with mood disorders.Reference Owens 6 , Reference Solmi, Pigato, Kane and Correll 46 , Reference Patterson-Lomba, Ayyagari and Carroll 47 , Reference Casey 62 , Reference Caroff 63
TD is associated with medical co-morbidities as well.Reference Caroff, Leong, Roberts, Berkowitz and Campbell 58 In particular, diabetes has been implicated in increasing the risk of TD in some but not all studies.Reference Miller, McEvoy and Davis 45 , Reference Caroff, Leong, Roberts, Berkowitz and Campbell 58 , Reference Ganzini, Casey, Hoffman and Heintz 64 However, this association and other medical co-morbidities may represent an artifact of the concomitant metabolic side effects of antipsychotics experienced by older and chronically treated patients rather than supporting diabetes as a risk factor for TD.
Pharmacogenetic studies of TD are preliminary but offer promise of identifying risk for TD.Reference Tsermpini, Redenšek and Dolžan 65 Association studies of TD have focused on polymorphisms of genes related to metabolism of antipsychotics, neurotransmitter receptors, transporter proteins, and genes involved in synaptic plasticity, oxidative stress, and inflammation. One interesting candidate is SLC18A2 which encodes the vesicular monoamine transporter-2, the target of VMAT2 inhibitors. Detection of SLC18A2 variants might allow identification of alterations in VMAT2 function informing the use of VMAT2 inhibitors and antipsychotics to prevent or treat TD. Studies to date have not yet provided consistent evidence that would support the implementation of genetic testing to predict TD in clinical practice.
The risk of TD differs among antipsychotics. Drugs classified as second-generation antipsychotics (SGAs) have consistently shown a reduced risk of TD compared with first-generation antipsychotics (FGAs).Reference Carbon, Hsieh, Kane and Correll 36 , Reference Carbon, Kane, Leucht and Correll 37 , Reference Casey 56 , Reference Correll, Leucht and Kane 66 -Reference Tenback, van Harten, Slooff, Belger and van Os 68 The true risk differential may not be as great as initially thought, ranging from one-quarter to two-thirds of the risk of FGAs.Reference Patterson-Lomba, Ayyagari and Carroll 47 , Reference Woods, Morgenstern and Saksa 69 -Reference Miller, Caroff and Davis 72 Several meta-analyses have shown that relative risk may have more to do with D2-receptor blocking potency of individual antipsychotics rather than the year a drug was introduced.Reference Correll and Schenk 73 , Reference Leucht, Wahlbeck, Hamann and Kissling 74 Patients receiving higher cumulative doses for longer duration are intuitively at greater risk,Reference Patterson-Lomba, Ayyagari and Carroll 47 , Reference Casey 56 , Reference Kane, Rifkin and Woerner 75 although clinicians should remain vigilant given that even short-term treatment with low doses may induce TD in vulnerable patients.Reference Zichlin, Mu, Leo and Ayyagari 76 So, for example, prolonged high doses of haloperidol predictably cause TD, whereas the risk of TD with clozapine is significantly diminished.Reference Woods, Morgenstern and Saksa 69 , Reference Kane, Woerner, Pollack, Safferman and Lieberman 77
The relative risk of long-acting injectable antipsychotics compared to oral agents is unclear but likely similar, with newer SGAs less likely than FGAs to cause TD in some but not all studies.Reference Kannarkat, Morley and Caroff 78 -Reference Saucedo Uribe, Carranza Navarro and Guerrero Medrano 81 Studies of long-acting injectable agents have suggested that guaranteed continuous administration of antipsychotics may actually decrease the risk of TD compared with intermittent treatment resulting from “drug holidays,” symptom-driven administration or patient nonadherence.Reference Jeste and Wyatt 82 -Reference Hogarty, Schooler, Ulrich, Mussare, Ferro and Herron 85
The effect of other prescription drugs or substance abuse on the risk of TD is less clear. Anticholinergic drugs have often been associated with TD. While anticholinergics acutely exacerbate TD symptoms and withdrawal may improve TD,Reference Caroff 63 , Reference Caroff, Gutman, Northrop, Leong, Berkowitz and Campbell 86 it remains unclear whether they are risk factors for the development of TD.Reference Owens 6 As they are commonly started or maintained in patients with drug-induced parkinsonism and other acute movement disorders, which are themselves associated with TD,Reference Tenback, van Harten, Slooff and van Os 87 , Reference Sachdev 88 the association between anticholinergics and TD may simply reflect people with predisposition to all movement disorders. The effects of nicotine and smoking on the risk and severity of TD are unclear and confounded by changes in plasma levels of antipsychotics induced by hydrocarbons in cigarette smoke.Reference Owens 6 , Reference Caroff, Gutman, Northrop, Leong, Berkowitz and Campbell 86 , Reference Quik, Boyd, Bordia and Perez 89 , Reference Meyer 90 Although other psychotropic agents often used concurrently with antipsychotics induce a variety of abnormal movements, for example, tremor, ataxia, or myoclonus, their influence on the risk of TD has not been well studied.Reference Casey 62 , Reference Friedman 91 While the development of TD-like movements with serotonin reuptake inhibitors or other antidepressants used as monotherapy is theoretically possible and has been reported in rare cases, whether irreversible movements can occur in the absence of dopamine receptor blockers is still controversial.Reference Friedman 91 , Reference D’Abreu and Friedman 92 Finally, drugs used in other medical specialties that have dopamine receptor blocking properties, for example, metoclopramide, prochlorperazine, flunarizine, or cinnarizine, are associated with the risk of TD and should not be overlooked.Reference Jhang, Huang and Nfor 93
Evidence-based strategies and tools for the diagnosis of patients with TD
Phenomenology
Although the symptoms of TD are mild and affect the orofacial region in 60% to 80% of cases,Reference Owens 6 manifestations can be complex taking a variety of phenomenological forms that can range widely in severity and impact (Table 1).Reference Owens 6 , Reference Hauser, Meyer and Factor 94 , Reference Factor 95 The variety of movements has generated discussion of whether the term “tardive dyskinesia” should be restricted to the classic oral-buccal-lingual movements as opposed to a more encompassing term such as “tardive syndromes.”Reference Hauser and Truong 25 , Reference Frei, Truong, Fahn, Jankovic and Hauser 96 TD movements can affect any striated or voluntary muscle group and are most often described as stereotyped or choreiform, but can appear as dystonia, akathisia, tics, or myoclonus. TD may have a sensory component as well, typically pain accompanying movements. The notion that these different forms represent phenotypic manifestations of the same underlying process is supported by the fact that they often occur concurrently in the same patient, for example, approximately 10% of patients with classic TD also have tardive dystonia.Reference Hauser, Meyer and Factor 94 , Reference van Harten, Hoek, Matroos, Koeter and Kahn 97 While it is unclear to what extent different forms of TD predict different treatment response or prognosis, it is critical for clinicians to be able to distinguish TD from acute and reversible drug-induced movement disorders which respond oppositely to TD (Table 2).Reference Hauser, Meyer and Factor 94 But it is important to note that acute movements can also co-exist in patients with TD raising challenging treatment questions.Reference van Harten, Matroos, Hoek and Kahn 50 In patients receiving antipsychotics, at least 20% to 30% may have two or more drug-induced movement disorders, and 13% and 5%, respectively, of those with TD may simultaneously have parkinsonism or akathisia.Reference van Harten, Matroos, Hoek and Kahn 50 , Reference Rekhi, Tay and Lee 98 , Reference Chouksey and Pandey 99
Table 1. Phenomenology of Tardive Dyskinesia

Table 2. Comparison of Tardive Dyskinesia and Drug-Induced Parkinsonism

Diagnostic criteria
Standardized diagnostic criteria for TD in DSM5-TR include a concise description of clinical signs and require exposure to antipsychotics of at least 3 months (1 month for patients over age 60) or occurring within 4 weeks of withdrawal from oral agents or 8 weeks from long-acting agents. 100 In ICD-10-CM, TD is formally coded as “drug-induced subacute dyskinesia” but is poorly defined and often miscoded under other and unspecified drug-induced movement disorders or neglected entirely in recorded databases.Reference Caroff, Leong, Roberts, Berkowitz and Campbell 58 , 101 , Reference Caroff, Mu, Ayyagari, Schilling, Abler and Carroll 102 The best known and widely accepted operationalized criteria are the Schooler-Kane research criteria which posit the presence of at least moderate movements in one area or mild in two areas on the Abnormal Involuntary Movement Scale (AIMS) that are not caused by another etiology and are associated with at least 3 months of antipsychotic exposure.Reference Schooler and Kane 103 Glazer et alReference Glazer, Morgenstern and Doucette 104 proposed a lower threshold for diagnosis, with movements meeting a total AIMS score of three with at least one area rated as mild. Use of higher diagnostic thresholds on the AIMS in past epidemiologic studies may have underestimated the true extent of TD since recent evidence suggests that even mild movements in one body area are diagnostic for TD.Reference Caroff, Citrome and Meyer 40 , Reference Caroff, Leong, Roberts, Berkowitz and Campbell 105 Basing diagnosis on the number of areas recorded on the AIMS also may be problematic because the orofacial region is overweighted and differentiating overflow movements between the jaw, lips, and tongue as distinct areas is challenging.Reference Caroff, Citrome and Meyer 40 , Reference Caroff, Leong, Roberts, Berkowitz and Campbell 105
Differential diagnosis
The most common movement disorders for clinicians to distinguish from TD are spontaneous dyskinesias and other drug-induced movement disorders. Unusual movements including compulsions, mannerisms, and stereotypies comprise a core symptom dimension of schizophrenia.Reference Factor, Lang and Weiner 24 , Reference Fenton 55 , Reference Owens, Johnstone and Frith 57 , Reference Hirjak, Thomann, Kubera, Wolf, Sambataro and Wolf 106 , Reference Ungvari, Caroff and Gerevich 107 People with depression or mania are similarly characterized by abnormal movements or catatonic signs.Reference Taylor and Abrams 108 Aging itself predisposes to abnormal movements, especially in the orofacial region and when edentulousness occurs.Reference Lieberman, Kane, Woerner and Weinhold 48 , Reference Owens, Johnstone and Frith 57 These observations prompted studies comparing the incidence of spontaneous dyskinesias to TD, suggesting that antipsychotics reveal or facilitate the appearance of abnormal movements in people who are naturally predisposed to their eventual occurrence.Reference Kane and Smith 13 , Reference Casey 109
Given the contrasting responses to treatment (Table 2), it is also important to differentiate acute reversible drug-induced movements, induced by use of illicit or prescription drugs, from TD. While the phenomenology between some forms of TD and acute movements, for example, dystonia or akathisia, may be similar, temporal correlation with antipsychotic treatment helps distinguish between them.Reference Owens 6 , Reference Factor, Lang and Weiner 24 , Reference Hauser, Meyer and Factor 94 , Reference Tarsy 110 , Reference Caroff and Campbell 111 TD is more likely to be delayed after antipsychotic treatment initiation, and often appears or worsens and can persist after drug dose reduction or discontinuation. Psychotropic drugs other than antipsychotics are also associated with abnormal movements.Reference Factor, Lang and Weiner 24 , Reference Casey 62 , Reference Friedman 91 , Reference Baizabal-Carvallo and Morgan 112 , Reference Revet, Montastruc, Roussin, Raynaud, Lapeyre-Mestre and Nguyen 113 Lithium, antidepressants, and anticonvulsants may cause tremors while antidepressants and stimulants may also cause or worsen akathisia, tics, and TD. However, in nearly all these cases, the movements are acute and reversible with drug discontinuation whereas dopamine-receptor blockers appear to be uniquely associated with persistent or irreversible movements.Reference D’Abreu and Friedman 92
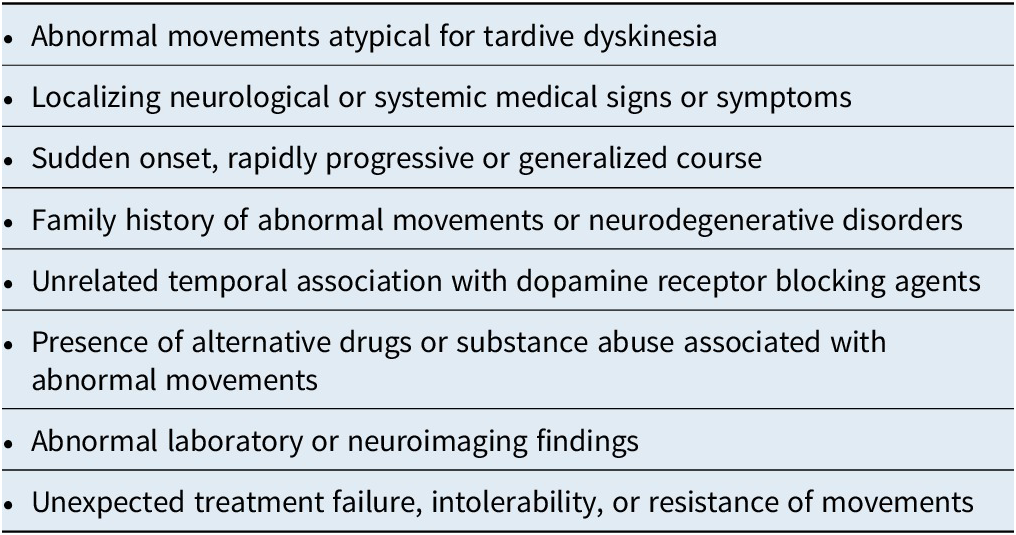
Finally, the differential diagnosis of movement disorders in general is extensive across the spectrum of pathological etiologies.Reference Owens 6 , Reference Factor 95 Although overlooking any of these neurological or systemic disorders could be tragic, they are mostly rare and irreversible, and are best evaluated in collaboration with medical or neurological consultants. Clues that may indicate an underlying etiology are worth keeping in mind (Table 3).Reference Caroff, Citrome and Meyer 40
Table 3. Clues to Alternative Etiologies in the Differential Diagnosis of Tardive Dyskinesia
Screening tools and policies
Regular screening to detect early signs of TD is critical. Among a number of rating instruments proposed for screening and monitoring, the AIMS achieved high interrater reliability and near-universal acceptance as the standard tool in clinical trials and epidemiology research, in clinical practice settings, and by regulatory agencies.Reference Guy 114 Based on a standardized examination, the AIMS scale measures the objective severity of abnormal movements across seven selected muscle groups ranging from normal to severe judged on the basis of quality, amplitude, frequency, anatomic distribution, and duration of abnormal movements observed during the examination.Reference Munetz and Schulz 115 , Reference Lane, Glazer, Hansen, Berman and Kramer 116
Opportunities to extend its usefulness have received renewed attention. For example, shortcomings of the AIMS include; whether a total summed score obscures the distribution of symptoms; the value of a single global score; and lack of consensus on a minimal clinically important difference to assess the significance of change scores.Reference Caroff, Leong, Roberts, Berkowitz and Campbell 105 , Reference Kane, Correll, Nierenberg, Caroff and Sajatovic 117 , Reference Stacy, Sajatovic and Kane 118 Awareness, incapacitation, and dental items have not been standardized and are less reliable. The AIMS is weighted toward orofacial movements and does not provide information about phenomenology which can have a major impact on prognosis as well as tolerability.Reference Bhidayasiri, Kane and Frei 119 While more comprehensive rating scales measuring all abnormal movements have been suggested for research or specialty settings, time constraints in a busy clinical practice support the need for simplified screening tools. Evidence suggests that the major reason for noncompliance with screening is lack of sufficient time.Reference Caroff, Leong, Roberts, Berkowitz and Campbell 105 Therefore, a number of efforts have been undertaken to validate brief screening tools or virtual procedures,Reference Caroff, Yeomans and Lenderking 120 -Reference Bera, Franey, Martello, Bron and Yonan 123 and to instruct patients and caregivers on self-examination to detect early signs.Reference McEvoy and Nierenberg 124 -Reference Munetz and Roth 126
Another area not covered by the AIMS is the impact of TD. The impact of TD is critical in determining how severity and changes in abnormal movements affect functioning and quality of life.Reference Caroff, Yeomans and Lenderking 120 , Reference Cutler, Caroff and Tanner 127 While severe TD may impair functioning in all affected people, even mild TD may have profound effects on an individual.Reference Ayyagari, Goldschmidt, Mu, Caroff and Carroll 128 Recent studies have documented the impact of TD and piloted the use of rating scales that incorporate functional measures. Additional evidence is needed on the predictive validity of AIMS scores in determining the effect of TD on functioning and quality of life.Reference Caroff, Leong, Roberts, Berkowitz and Campbell 105
Finally, a screening tool is only as effective as the reliability with which it is applied. Guidelines and institutional policies vary in how often screening with AIMS should occur.Reference Keepers, Fochtmann and Anzia 129 Previous policies mandating screening on an annual basis are insufficient and likely to miss a significant number of cases precluding early intervention and potential reversal.Reference Caroff, Citrome and Meyer 40 Questions about TD and visual inspection for abnormal movements should be added to screening assessments of side effects at every clinical visit.
Formulating individualized treatment plans for patients with tardive dyskinesia
Once TD is diagnosed, a logical sequence of management decisions follows.Reference Caroff 63 , Reference Citrome 130 First, assessment of the severity, distribution, and phenomenology of TD should be conducted by documenting a complete AIMS examination. Assessment of the functional impact should be conducted by asking concrete, descriptive questions on awareness and impact on self-esteem and limitations in health, social and occupational functioning. Second, a differential diagnosis should be considered to rule out spontaneous dyskinesias or other acute drug-induced movements, with specialist consultation if the diagnosis is uncertain or other rare neurological disorders are suspected. Once TD is confirmed, a discussion should take place with patients and caregivers to inform them of the diagnosis, prognosis, and treatment options.
It is important to consider the natural course of TD in deciding on subsequent treatment decisions. Abnormal movements fluctuate with activation, anxiety, relaxation, inattention, or distraction, they can be suppressed by voluntary action, and they disappear during sleep. TD severity can fluctuate over the course of a day or between clinical visits. In patients with TD at baseline randomized to receive SGAs in one large-scale trial, 76% met the criteria for TD at some or all subsequent visits, 24% did not meet the criteria at any visit, 32% showed more than 50% improvement in TD, while 7% showed more than 50% worsening of AIMS scores.Reference Caroff, Davis and Miller 59 The relationship to treatment is informative, as 5% to 67% of patients may have TD that is covert or masked by ongoing antipsychotic treatment and only becomes apparent after drug discontinuation.Reference Caroff, Ungvari and Cunningham Owens 7 Withdrawal dyskinesia is defined by a movement first observed after treatment cessation that subsides within 4-12 weeks after antipsychotic discontinuation. 100 Withdrawal dyskinesias that subside after drug discontinuation do not necessarily require specific treatment. The distinction between withdrawal dyskinesia and incipient TD may be arbitrary and more likely, withdrawal dyskinesias are on a continuum with persistent TD. In other words, early TD may be reversible after drug discontinuation, but may become irreversible after a variable period of time if antipsychotics are continued.
Subsequent prescribing decisions begin with re-evaluating antipsychotic treatment. Maintenance of current treatment may be justified in patients with severe psychiatric illness at high risk for relapse who are stable on current antipsychotic therapy but have mild, localized TD with minimal subjective impact. In most cases, TD is not progressive even with continued antipsychotic treatment, although symptoms may worsen in some cases.Reference Caroff, Davis and Miller 59 Patients should provide informed consent and should be carefully monitored for any signs of progression.
Ideally, antipsychotics could be tapered off. In patients without an underlying psychotic disorder, for example, those who develop TD while taking adjunctive dopamine antagonists for depression, antipsychotic treatment could be safely tapered.Reference Casey 62 However, patients with schizophrenia, bipolar disorder, or psychotic depression may incur a significant risk of relapse and require ongoing maintenance treatment.Reference Gilbert, Harris, McAdams and Jeste 131 More research on the early mechanisms underlying onset leading to permanence of TD are needed. Meanwhile, early detection and reconsidering antipsychotic treatment for patients who could be safely treated by other means is prudent to account for the possibility of reversible TD after drug discontinuation, whereas established TD is unlikely to resolve.
However, complete and permanent reversibility beyond the withdrawal period is controversial and may be uncommon. Early studies suggested that after withdrawal from FGAs, approximately 33% to 53% of patients experience worsening of dyskinesias initially, while 36% to 55% may show improvement over time.Reference Egan, Apud and Wyatt 132 In a meta-analysis, Soares and McGrath reported that 37.3% of patients assigned to placebo across studies showed some improvement of TD, but concluded that evidence was insufficient to support antipsychotic drug cessation or dose reduction in view of the risk for psychotic relapse.Reference Soares and McGrath 133 While recent surveys suggest as few as 2% to 5% of patients show resolution of TD without specific treatment even when followed-up for a few years after antipsychotic withdrawal,Reference Zutshi, Cloud and Factor 134 , Reference Glazer, Moore, Schooler, Brenner and Morgenstern 135 earlier studies reported remission a few months to years after drug cessation in 50% to 75% of patients provided that TD was detected early.Reference Jeste, Potkin, Sinha, Feder and Wyatt 83 , Reference Quitkin, Rifkin, Gochfeld and Klein 136 -Reference Casey, Gerlach, Casey and Gardos 138 These early reports suggest that further studies of antipsychotic cessation in nonpsychotic patients as a management option for new-onset cases of TD are needed.
Another option is the dose reduction of antipsychotics. Evidence is mixed on whether dose reduction improves TD which in fact may temporarily worsen on decreased dosages.Reference Kane, Woerner, Sarantakos, Kinon, Lieberman, Casey and Gardos 139 -Reference Bhidayasiri, Fahn and Weiner 141 Recent evidence suggests that dose reduction like drug cessation may contribute to psychotic relapses and possible re-hospitalization, further limiting this treatment option for TD without additional evidence.Reference Caroff, Mu, Ayyagari, Schilling, Abler and Carroll 102
If maintenance treatment is necessary or if a patient requests a change from the agent that caused TD, switching to more potent antipsychotics or FGAs may temporarily suppress TD in up to 67% of patients, although limiting remission of TD and exacerbating parkinsonism and other acute drug-induced movements.Reference Egan, Apud and Wyatt 132 , Reference Mentzel, Bakker and van Os 142 -Reference Kazamatsuri, Chien and Cole 144 Several studies of SGAs have shown a reduction in TD severity, with some studies showing greater suppression, lesser suppression, or no difference compared with FGAs.Reference Caroff, Davis and Miller 59 , Reference Factor 95 , Reference Bhidayasiri, Fahn and Weiner 141 , Reference Tarsy, Baldessarini and Tarazi 145 In fact, studies of SGAs in suppressing TD have shown approximately a 3-4 point average decrease in total AIMS severity scores which is comparable to decreases in recent trials of VMAT2 inhibitors.Reference Simpson, Lee and Shrivastava 15 , Reference Caroff, Davis and Miller 59 , Reference Mentzel, Bakker and van Os 142 , Reference Mentzel, van der Snoek and Lieverse 146 -Reference Bai, Yu and Lin 149 Among SGAs, some studies have highlighted evidence that clozapine significantly reduces symptoms of moderate to severe TD, especially dystonic movements, suggesting that it should be considered an alternative antipsychotic in patients with TD who may not be responding to other approaches.Reference Mentzel, van der Snoek and Lieverse 146 , Reference Tamminga, Thaker, Moran, Kakigi and Gao 148 , Reference Lieberman, Saltz, Johns, Pollack, Borenstein and Kane 150 , Reference Pardis, Remington, Panda, Lemez and Agid 151 However, switching any antipsychotics may incur the risk of destabilizing psychiatric symptoms in an otherwise stable patient.Reference Ayyagari, Thomason, Mu, Philbin and Carroll 152
If anticholinergic drugs have been prescribed, a decision about continuation or tapering should be considered. Anticholinergic drugs are not effective as a treatment for TD; in fact, they may worsen TD acutely, such that improvement in TD severity ratings have been noted in up to 60% of patients withdrawn from these agents.Reference Egan, Apud and Wyatt 132 , Reference Soares-Weiser and Fernandez 143 , Reference Greil, Haag, Rossnagl and Ruther 153 -Reference Simpson 155 But caution should be taken in reducing anticholinergics if acute drug-induced movements (eg, parkinsonism, dystonia) or tardive dystonia are present as these disorders could re-emerge or worsen after withdrawal.Reference Simpson 155 Amantadine may be a reasonable alternative to anticholinergics in patients with both TD and parkinsonism, especially if they are at risk for adverse anticholinergic effects; it is now marketed in a long-acting formulation and evidence suggests it may have beneficial effects on both dyskinesias and parkinsonism.Reference Ward and Citrome 154 , Reference Caroff, Jain and Morley 156 It is also helpful to recall that some studies have suggested that patients with TD are heterogeneous in response to pharmacological agents.Reference Casey and Denney 10 , Reference Lieberman, Pollack, Lesser and Kane 17
If TD remains a significant problem once antipsychotic treatment is optimized, specific antidyskinetic treatment with VMAT2 inhibitors should be considered. Numerous and mostly flawed past clinical trials of agents engaging diverse pharmacological targets yielded inconclusive or negative results.Reference Bhidayasiri, Fahn and Weiner 141 , Reference Soares-Weiser and Fernandez 143 , Reference Caroff, Campbell and Carroll 157 -Reference Soares-Weiser, Rathbone, Ogawa, Shinohara and Bergman 159 In contrast, recent trials have provided robust evidence on the efficacy and tolerability of valbenazine and deutetrabenazine (Table 4). Like tetrabenazine, these drugs act to deplete dopamine and thereby reduce the severity of TD by inhibiting VMAT2 in nerve terminals, which ordinarily protects dopamine from the metabolic breakdown by transporting monoamines into protective vesicles. Despite a paucity of controlled trials, tetrabenazine is efficacious for the treatment of TD based on observational studies, but is limited by a short half-life and side effects of sedation, parkinsonism, akathisia, and depression.Reference Jankovic and Clarence-Smith 22 , Reference Caroff, Aggarwal and Yonan 160 , Reference Scorr and Factor 161 Valbenazine and deutetrabenazine were designed with improved pharmacokinetics to allow for a longer half-life and reduced fluctuations associated with differences in genetic metabolic rate and drug interactions. Rigorous short and long-term trials have confirmed that both drugs are more effective than placebo in reducing severity of TD and are likely better tolerated than tetrabenazine.Reference O’Brien, Jimenez and Hauser 26 -Reference Fernandez, Factor and Hauser 29 , Reference Citrome 162 -Reference Lindenmayer, Verghese and Marder 165 Although both are effective compared to placebo, valbenazine has the advantage of simple titration and once-a-day dosing, whereas deutetrabenazine may allow for smaller increments of titration. Variability of response to VMAT2 inhibitors based on phenomenology has yet to be tested, but earlier trials suggested that both tardive dystonia and tardive akathisia also improve with these drugs.Reference Burke, Kang, Jankovic, Miller and Fahn 166 -Reference Kiriakakis, Bhatia, Quinn and Marsden 168
Table 4. Comparison of VMAT2 Inhibitors

If TD does not respond to one VMAT2 inhibitor or side effects emerge, a second VMAT2 inhibitor could be tried. Among other agents that have not met the threshold of evidence to justify approval, some may have efficacy for subgroups of patients with TD. Cholinergic agents (acetylcholine precursors, cholinesterase inhibitors) have been tried with inconclusive results, but emerging drugs targeting specific cholinergic receptors may be worth exploring.Reference Caroff, Gutman, Northrop, Leong, Berkowitz and Campbell 86 , Reference Caroff, Walker and Campbell 169 GABA-ergic drugs have questionable benefits in reducing TD and are limited by side effects.Reference Bhidayasiri, Fahn and Weiner 141 , Reference Soares-Weiser and Fernandez 143 , Reference Alabed, Latifeh, Mohammad and Rifai 170 For example, although clonazepam has been rated as probably improving TD and is commonly used, it is limited by its potential for abuse and significant hazards of sedation, ataxia, and cognitive impairment, especially in older adults.Reference Caroff, Campbell and Carroll 157 Evidence that dopaminergic blockade results in excess dopamine turnover and oxidative free radical formation formed the rationale for trials of antioxidants albeit with limited support to date.Reference Bhidayasiri, Fahn and Weiner 141 For example, although vitamin E appeared promising as a treatment for TD in early pilot studies, a subsequent definitive large-scale randomized controlled trial failed to show a significant effect on TD compared to placebo.Reference Adler, Rotrosen and Edson 171 Amantadine, an N-methyl-D-aspartate receptor antagonist with dopaminergic and anticholinergic properties, has undergone preliminary testing as a treatment for TD and may have value in patients with concurrent TD and parkinsonism.Reference Bhidayasiri, Fahn and Weiner 141 , Reference Ward and Citrome 154 , Reference Caroff, Jain and Morley 156 In tardive dystonia, some evidence suggests that anticholinergics, botulinum toxin, or neurosurgical procedures may be beneficial.Reference Factor 95 , Reference Burke, Fahn and Jankovic 167 , Reference Kiriakakis, Bhatia, Quinn and Marsden 168
Looking ahead
The explosive growth in promotion and prescribing of antipsychotics is likely to continue, resulting in more cases of TD. There is a compelling need for education among practitioners about conservative use of antipsychotics, the risks of TD, and screening and monitoring policies and instruments. In addition, research and development of antipsychotics that target novel brain targets (trace amine-associated receptor 1 agonists, serotonin agonists and serotonin/opioid antagonists, muscarinic receptor agonists) that lack dopamine receptor affinity may offer promise as effective antipsychotics without movement disorder liability.Reference Kannarkat, Morley and Caroff 78
Meanwhile, VMAT2 inhibitors are likely to transform the treatment of TD and renew interest in TD research.Reference Meyer 172 Although trials of VMAT2 inhibitors offer clear evidence of efficacy and tolerability in suppressing TD, their impact on real-world outcomes merits additional investigation. There are as yet no head-to-head prospective trials comparing VMAT2 inhibitors, SGAs, or other antidyskinetic agents.Reference Caroff, Aggarwal and Yonan 160 , Reference Aggarwal, Serbin and Yonan 173 In addition, continuing studies of the social, psychological, and economic impact of TD are likely to provide crucial evidence to better inform cost–benefit treatment decisions in the near future.Reference Atlas, Agboola and Curfman 174
The interaction between the VMAT2 inhibitors and antipsychotics has also raised interesting questions.Reference Lindenmayer, Burke, Tsuboyama, Chahal and Grewal 175 For example, adding VMAT2 inhibitors early in treatment for first-episode patients may allow lower doses of antipsychotics to be used possibly reducing the risk of TD. However, dopamine depletion, like denervation, may theoretically add to the risk of TD from antipsychotic-induced dopamine receptor blockade.Reference Caroff 63 , Reference LeWitt 176 In addition, by suppressing TD symptoms without blocking dopamine receptors, these agents might allow patients who do not require maintenance antipsychotics to tolerate drug withdrawal and therefore facilitate natural reversal of the mechanisms underlying TD over time.Reference Hauser, Barkay and Fernandez 177 As suggestive evidence, approximately 25% to 30% of patients enrolled in long-term extension studies of valbenazine continued to show a reduction of TD even 4 weeks after withdrawal from valbenazine.Reference Caroff, Lindenmayer, Farahmand, Burke and Siegert 178 In patients without the need for maintenance antipsychotics, long-term withdrawal studies may provide answers in the future.
Conclusion
The availability of novel VMAT2 inhibitors has transformed the treatment of TD. These findings need to be tested in real-world settings over the long term and taken into consideration as part of a broader, practical treatment algorithm that provides a stepwise approach to the management of patients affected by TD. Availability of these treatments may also rekindle interest in TD and the need for re-education and implementation of standardized practice guidelines. Additional research should include investigations of underlying pathophysiological mechanisms, variations in phenomenology, genetic predisposition, and the efficacy of other novel antipsychotic and antidyskinetic drugs, especially those targeting non-dopaminergic mechanisms, with the ultimate goal of achieving prevention, reversibility, and remission of TD symptoms.
Financial Support
This activity is supported by an unrestricted educational grant from Neurocrine Biosciences to the Neuroscience Education Institute (NEI).
Disclosures
Dr. Caroff served as a consultant for Neurocrine Biosciences and Adamas Pharmaceuticals. Dr. Caroff also received separate research grants from Neurocrine Biosciences and Eagle Pharmaceuticals.








