Introduction
Diarrhoea caused by parasitic protozoa is the second leading cause of death among children worldwide (Kotloff et al., Reference Kotloff, Nataro, Blackwelder, Nasrin, Farag, Panchalingam, Wu, Sow, Sur, Breiman, Faruque, Zaidi, Saha, Alonso, Tamboura, Sanogo, Onwuchekwa, Manna, Ramamurthy, Kanungo, Ochieng, Omore, Oundo, Hossain, Das, Ahmed, Qureshi, Quadri, Adegbola, Antonio, Hossain, Akinsola, Mandomando, Nhampossa, Acácio, Biswas, O'Reilly, Mintz, Berkeley, Muhsen, Sommerfelt, Robins-Browne and Levine2013). In most cases, the specific cause of diarrhoea remains unidentified, which can be caused by various intestinal pathogens, including viruses, bacteria and protozoan parasites (Caner et al., Reference Caner, Zorbozan, Tunalı, Kantar, Aydoğdu, Aksoylar, Gürüz and Turgay2020; David et al., Reference David, Fonseca, de Carvalho, Marinho, Duro, Komninakis and Souto2022; Kotloff, Reference Kotloff2022). Acute diarrhoea is mainly caused by viral infections, while persistent chronic diarrhoea is more commonly caused by parasitic infections, which are increasingly recognized as the main cause of diarrhoeal diseases (Lima and Guerrant, Reference Lima and Guerrant1992). It has been established that Giardia sp., Entamoeba sp., Blastocystis sp., Cryptosporidium sp. and other parasitic infections can cause diarrhoea. Although they share similar clinical symptoms, it is important to note that the optimal approaches for their diagnosis, treatment and prevention can differ. Among them, Blastocystis sp. is one of the most common intestinal protozoans detected in humans (Kaewjai et al., Reference Kaewjai, Tonsomboon, Pawiwongchai and Prommano2023). Therefore, it is very important to establish a novel method for rapid and accurate diagnosis of Blastocystis sp.
At present, the main diagnostic methods for Blastocystis sp. are microscopic detection, immunological detection and molecular biological detection. The microscopic detection of Blastocystis sp. mainly includes fecal smear microscopy, staining microscopy and in vitro culture. However, the fecal smear method and staining microscopy are prone to false negatives, while in vitro culture is complicated and takes a long time to detect (Zamani et al., Reference Zamani, Khademvatan, Tappeh, Diba and Abasi2021). Immunofluorescence and enzyme-linked immunosorbent assay has low specificity and are currently limited to epidemiological and serological studies. Molecular biological diagnosis is one of the conventional diagnostic methods for Blastocystis sp. Conventional polymerase chain reaction (cPCR) has become increasingly common and popular in identifying species. This method has high sensitivity and specificity, especially in the differential diagnosis between species, and has also been used in the genotyping and differential diagnosis of Blastocystis sp. (Yoshikawa et al., Reference Yoshikawa, Wu, Kimata, Iseki, Ali, Hossain, Zaman, Haque and Takahashi2004; Stensvold et al., Reference Stensvold, Brillowska-Dabrowska, Nielsen and Arendrup2006; Domínguez-Márquez et al., Reference Domínguez-Márquez, Guna, Muñoz, Gómez-Muñoz and Borrás2009; Khademvatan et al., Reference Khademvatan, Masjedizadeh, Yousefi-Razin, Mahbodfar, Rahim, Yousefi and Foroutan2018). However, cPCR requires particular equipment, including a thermal cycler, personnel with appropriate skills and training to conduct DNA-based diagnostic assays, and suitable cold storage to preserve the heat-sensitive reagents. This limitation hinders the widespread use and application in low-resource settings. Therefore, cPCR detection is usually only used in centralized clinical laboratories, highlighting the need for a faster, simpler and more accurate detection method that can be readily implemented in point of care settings.
Recombinase polymerase amplification (RPA), a isothermal amplification technique for nucleic acids, was developed in the UK (Piepenburg et al., Reference Piepenburg, Williams, Stemple and Armes2006). It has been established that recombinases form nucleoprotein complexes with oligonucleotide primers and search for homologous sequences in the DNA in RPA reactions. Upon recognition of a specific homologous sequence, the RPA technique initiates the template's synthesis and then amplifies the reverse strand through isothermal chain replacement by polymerase, thus allowing amplification of double-stranded DNA without thermal or chemical denaturation. The RPA test can be done in an hour or less with a similar reagent cost to cPCR but with a lower labour cost and acceptable sensitivity and specificity levels (Mota et al., Reference Mota, Guimarães, Gandarilla, Filho, Brito and Mariúba2022). In recent years, this technique has been successfully applied to parasite diagnosis in many cases, including Giardia duodenalis (Crannell et al., Reference Crannell, Cabada, Castellanos-Gonzalez, Irani, White and Richards-Kortum2015), Entamoeba histolytica (Nair et al., Reference Nair, Rebolledo, White, Crannell, Richards-Kortum, Pinilla, Ramírez, López and Castellanos-Gonzalez2015) and Leishmania Viannia spp. (Saldarriaga et al., Reference Saldarriaga, Castellanos-Gonzalez, Porrozzi, Baldeviano, Lescano, de Los Santos, Fernandez, Saravia, Costa, Melby and Travi2016). However, there is currently no RPA method available for the detection of Blastocystis sp.
This study aimed to develop an RPA detection method targeting the small subunit ribosomal RNA (SSU rRNA) gene of Blastocystis sp. and evaluate its sensitivity, specificity, stability and clinical efficacy.
Materials and methods
DNA sample used in this study
A total of 8 intestinal pathogens were used in this study, including Blastocystis sp., Staphylococcus aureus, Escherichia coli, Dientamoeba fragilis, Trichuris trichiura, Ascaris lumbricoides, Giardia lamblia and Enterobius vermicularis. Among them, the bacterial samples were from the strain bank of Pathogenic Biology, Xinxiang Medical University. Blastocystis sp., D. fragilis, T. trichiura and G. lamblia were isolated, cultured and enriched from feces. Ascaris lumbricoides and E. vermicularis were isolated directly from feces. The DNA of all pathogens was extracted by DNA extraction kit (Qiagen, Hilden, Germany) and identified by PCR amplification and sequencing. All identified DNA samples were stored at −20°C until used.
Primer design
The RPA primers were designed according to the SSU rRNA gene sequence of Blastocystis sp. provided by the National Center for Biotechnology Information (NCBI), accession numbers: KR262943. The cPCR primers used in this study were obtained from a previous study by Scicluna et al. (Reference Scicluna, Tawari and Clark2006). All primers were synthesized by Sangon Bioengineering Co., Ltd (Sangon, Shanghai, China). The sequence information of the primers is shown in Table 1.
Table 1. Primers used in this study

F, forward; R, reverse.
Primer screening for RPA assay
Three pairs of RPA primers designed in this study were selected for the RPA test, and the best pair of primers was screened according to the agarose gel electrophoresis results.
Development of the RPA assay
The RPA assay was performed using the TwistAmp® Basic kit (TwistDx, Cambridge, UK) according to the manufacturer's instructions. Briefly, after the reaction mix (rehydration buffer, 29.5 μL; forward primer, 2.4 μL; reverse primer, 2.4 μL; nuclease-free water, 8.2 μL; 42.5 μL) was prepared, it was added to a basic freeze-dried powder and thoroughly mixed. Subsequently, 5 μL of DNA template and 2.5 μL magnesium acetate solution were added to the suspension. The reaction tubes were incubated in a water bath at 37°C for 15 min. After the incubation, the amplified products were observed by agarose gel electrophoresis.
Optimum reaction conditions for the RPA assay
The RPA assay was performed using the genomic DNA of Blastocystis sp. Seven reaction times (5, 10, 15, 20, 25, 30 and 35 min) and 7 temperatures (25, 30, 35, 37, 39, 41 and 45°C) were tested to determine the optimum reaction conditions for the RPA assay.
The minimum detection limit of the RPA assay
The genomic DNA of Blastocystis sp. was subjected to a 10-fold gradient dilution to obtain concentrations of 100 ng μL−1, 1 ng μL−1, 10 pg μL−1, 100 fg μL−1, 1 fg μL−1 and 10 ag μL−1. Each diluted DNA sample (5 μL) was used as a template and amplified using the RPA assay. In addition, cPCR was used to amplify the serially diluted DNA, and the minimum detection limit between RPA and cPCR was compared. For the cPCR amplification, a 25 μL reaction system was prepared, containing 12.5 μL of 2 × Taq Plus Master Mix, 8.5 μL nuclease-free water, 1 μL forward primer (10 μM), 1 μL reverse primer (10 μM) and 2 μL DNA. The thermal cycler (Thermo Fisher Scientific, Waltham, USA) settings were as follows: 94°C for 5 min (initial denaturation); 30 cycles at 94°C for 45 s (denaturation), 59°C for 1 min (annealing) and 72°C for 1 min (extension); 72°C for 10 min (final extension).
Specificity of the RPA assay
For specificity analysis, the genomic DNA of Blastocystis sp. and 7 non-target pathogens (S. aureus, E. coli, D. fragilis, T. trichiura, A. lumbricoides, G. lamblia and E. vermicularis) were used as templates for the RPA and cPCR assays.
Repeatability evaluation of the RPA assay
To confirm the repeatability of the developed RPA systems, genomic DNA at concentrations of 20, 4, 2 and 1 ng μL−1 were used. Five independent RPA tests were performed on 5 days under the same conditions.
Validation in clinical samples
The performance of the RPA assay to detect Blastocystis sp. was validated by 40 clinical fecal samples from the Third Affiliated Hospital of Xinxiang Medical University. The fecal samples were tested by the RPA assay and compared with the cPCR assay to ensure there were no instances of non-specific amplification.
Results
Primer screening for the RPA assay
The target genes amplified by 3 pairs of primers, RPA-1-281-F/R, RPA-2-175-F/R and RPA-3-232-F/R, were expected to have sizes of 281, 175 and 232 bp, respectively. The results were shown in Fig. 1. The target band was not amplified by RPA-2-175-F/R. Although RPA-3-232-F/R and RPA-1-281-F/R both obtained the correct target bands, only RPA-1-281-F/R was free of impurity bands. Therefore, RPA-1-281-F/R was chosen as the primer for RPA assay.

Figure 1. Primer screening. Three pairs of RPA primers were selected for the RPA test. Lane M: DL2000, lane 1: RPA-1-281-F/R, lane 2: RPA-2-175-F/R, lane 3: RPA-3-232-F/R, lane N: negative control.
Optimized RPA conditions
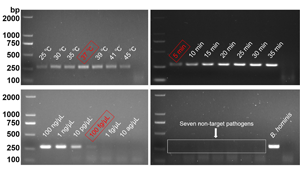
The optimal RPA conditions were determined by the brightness of target bands on agarose gel electrophoresis. At an incubation temperature of 37°C, the target bands could be detected within 5 min. With increased incubation time, the brightness of the band increased. In this study, 30 min was selected as the final incubation time of the RPA assay (Fig. 2). When the incubation time was set as 30 min, the target bands were detected in the 25–45°C temperature range. Among them, 37°C had the highest amplification efficiency (Fig. 3).

Figure 2. Optimum reaction time. Lane 1: 5 min, lane 2: 10 min, lane 3: 15 min, lane 4: 20 min, lane 5: 25 min, lane 6: 30 min, lane 7: 35 min, lane N: negative control.

Figure 3. Optimum reaction temperature. Lane 1: 25°C, lane 2: 30°C, lane 3: 35°C, lane 4: 37°C, lane 5: 39°C, lane 6: 41°C, lane 7: 45°C, lane N: negative control.
The minimum detection limit of the RPA assay
Six genomic DNA dilutions were detected by the RPA and cPCR assays, respectively, and the results are shown in Fig. 4. The minimum detection limit of RPA and cPCR assays was 100 fg μL−1, indicating that the detection method established in this study had high sensitivity.

Figure 4. The minimum detection limit of RPA assay. Different concentrations of DNA were used for the RPA assay and cPCR assay. (A) The minimum detection limit of RPA assay. (B) The minimum detection limit of cPCR assay. Lane 1: 100 ng μL−1, lane 2: 1 ng μL−1, lane 3: 10 pg μL−1, lane 4: 100 fg μL−1, lane 5: 1 fg μL−1, lane 6: 10 ag μL−1, lane N: negative control.
Specificity of the RPA assay
As shown in Fig. 5, Blastocystis sp. and 7 other non-target pathogens could be accurately distinguished by the RPA assay. The specificity of the RPA assay was consistent with the cPCR assay, indicating that the RPA assay has high specificity.

Figure 5. Specificity analysis. The genomic DNA of Blastocystis sp. and 7 non-target pathogens were used as templates for RPA assay and cPCR assay. (A) Specificity analysis of RPA assay. (B) Specificity analysis of cPCR assay. Lane 1: Enterobius vermicularis, lane 2: Ascaris lumbricoides, lane 3: Dientamoeba fragilis, lane 4: Trichuris trichiura, lane 5: Giardia lamblia, lane 6: Escherichia coli, lane 7: Staphylococcus aureus, lane P: positive control, lane N: negative control.
Repeatability evaluation of the RPA assay
As shown in Table 2, the 5 test results were completely consistent. Besides, there was no false-negative or false-positive result, indicating the good repeatability of the RPA assay.
Table 2. Repeatability evaluation of RPA assay

Performance validation with clinical samples
Forty clinical samples were tested by the RPA and cPCR assays, respectively (Fig. 6). The RPA test identified that 9 samples were positive for Blastocystis sp., and 31 were negative. These results were consistent with cPCR results, indicating high sensitivity and specificity of RPA assay. Therefore, the RPA assay proved to be a highly effective method for detecting Blastocystis sp.

Figure 6. Performance validation with clinical samples. The 40 clinical fecal samples were tested by RPA assay and cPCR assay to check for any non-specific amplification. (A) and (B) were RPA assays. (C) and (D) were cPCR assays. Lane 1–40: clinical fecal samples, lane P: positive control, lane N: negative control.
Discussion
It has been established that Blastocystis sp. exhibits significant genetic diversity in the SSU rRNA gene, which allows all the subtypes to be identified and can be used for large-scale epidemiological investigations (Scicluna et al., Reference Scicluna, Tawari and Clark2006). Therefore, the SSU rRNA gene of Blastocystis sp. was selected as the target sequence in this study. The results showed that the RPA method could detect Blastocystis sp., and there was no cross-reaction with 7 non-target pathogens in the gut, indicating that the method had good specificity. The minimum detection limit test results showed that the minimum detection limit of Blastocystis sp. genomic DNA was 100 fg μL−1, consistent with the minimum detection limit of the cPCR assay. The RPA assay could be carried out at 25–45°C, indicating its lower temperature requirements and wider detection range, conducive to the detection of Blastocystis sp. in low-resource settings (Abd et al., Reference Abd El Wahed, Patel, Faye, Thaloengsok, Heidenreich, Matangkasombut, Manopwisedjaroen, Sakuntabhai, Sall, Hufert and Weidmann2015). In addition, the target bands appeared after a reaction time of 5 min. When the reaction time was 30 min, the target bands became clearer, suggesting the optimal reaction time was 30 min. Compared with traditional detection methods or general molecular detection techniques (such as cPCR or loop-mediated isothermal amplification), the RPA assay significantly reduces detection time, leading to improved timeliness of results (Fang et al., Reference Fang, Liu, Zhang, Lin, Xu and Luo2011; Herrero et al., Reference Herrero, Vieites and Espiñeira2011).
Unlike PCR, which includes a high-temperature denaturation step to prevent non-specific amplification, the isothermal nature of RPA reactions makes them more susceptible to the formation of primer dimers. Primer dimers are unintended products formed when primers anneal to each other instead of the target sequence, leading to reduced amplification efficiency and may affect the test results in case of low or absent template concentration (Özay and McCalla, Reference Özay and McCalla2021). Therefore, 3 RPA primers were designed with the SSU rRNA gene as the target sequence in this study. RPA-1-281-F/R was selected by agarose gel electrophoresis as the best primer pair for detecting Blastocystis sp.
Higher sensitivity increases the likelihood of false-positive results during molecular biological detection. Therefore, 5 independent repeatable experiments were performed in this study, and the results showed that the RPA method established in this study ensured sensitivity while preventing false positives by optimizing the design of primers, reaction conditions and the entire reaction system. Meanwhile, the results of 40 clinical samples were consistent with the results of cPCR, indicating that the results of this method are highly credible and have good application prospects.
In summary, we constructed an isothermal amplification method for Blastocystis sp. nucleic acid using RPA technology. This method offers advantages, such as faster detection, simplicity in execution and high sensitivity and specificity rates, making it highly applicable for rapid Blastocystis sp. detection.
Data availability statement
The datasets supporting the findings of this article are included within the article and its additional files.
Acknowledgements
We would like to thank the Third Affiliated Hospital of Xinxiang Medical University for supporting this study.
Author contributions
X. M., S. W. and Z. Z. contributed to the design of this study. X. M., C. S., L. J. and S. Z. prepared the experimental samples and performed the experimental procedures. X. M., S. W., Z. Z., X. T. and Z. Y. analysed and interpreted the data. X. M. and S. W. drafted the manuscript. All authors read and approved the final manuscript.
Financial support
This work received support from the Natural Science Foundation of Henan Province (No. 232300421313), the Training Plan for Young Backbone Teachers in Universities of Henan Province (No. 2021GGJS101), the Doctoral Scientific Research Activation Foundation of Xinxiang Medical University (No. XYBSKYZZ202140), the Research Innovation Project of College Students of Xinxiang Medical University (Nos. xyxskyz201907 and xyxskyz202120). Finally, we would like to thank the Third Affiliated Hospital of Xinxiang Medical University for their support of this study.
Competing interests
None.
Ethical standards
Not applicable.